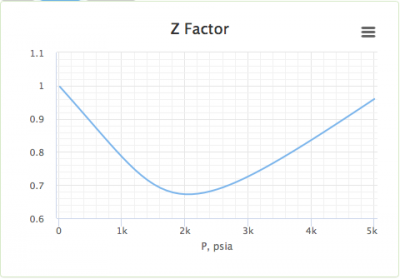
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Gas - Wikipedia
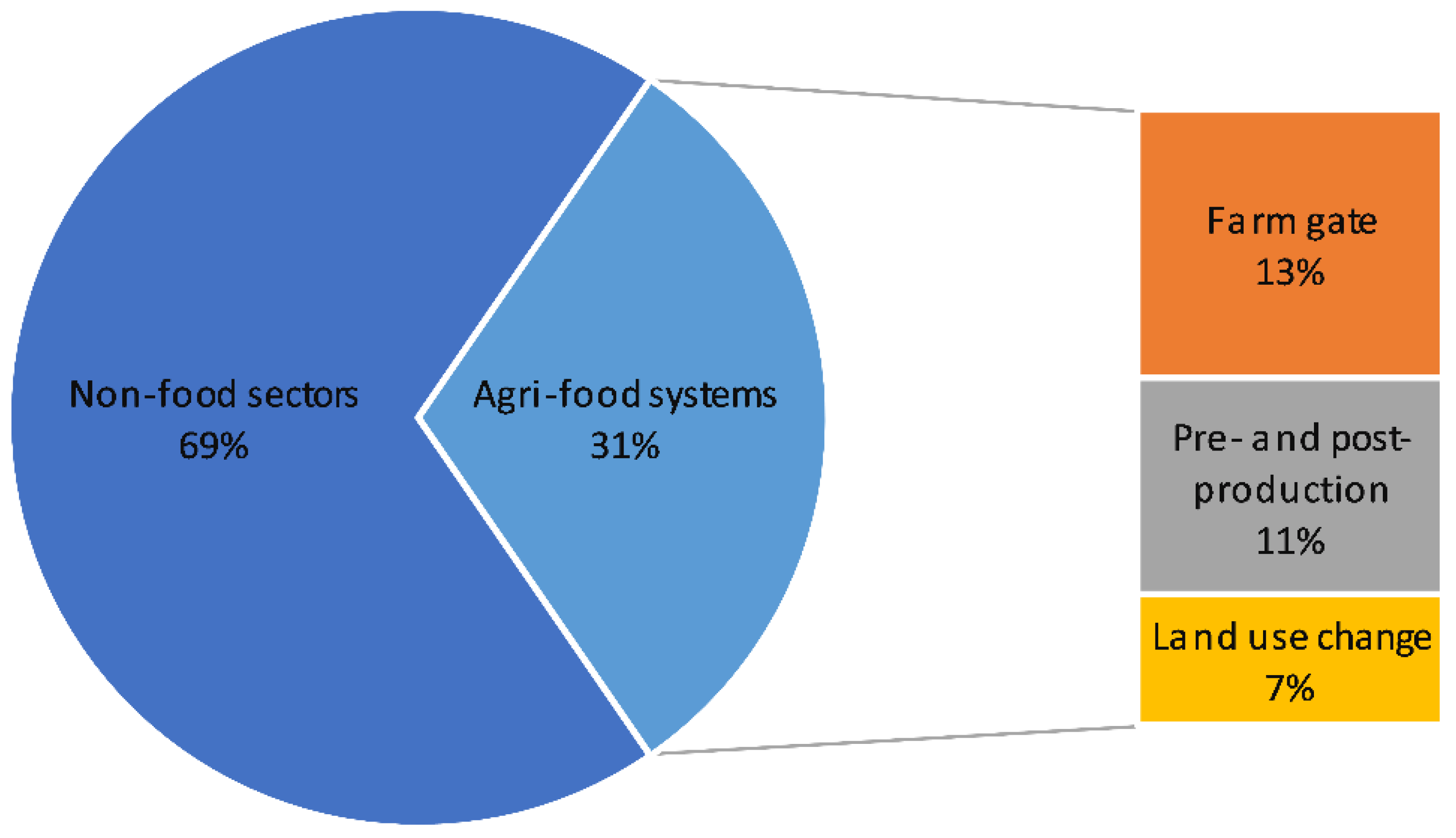
Agriculture, Free Full-Text

Water Phase Diagram, Comparisons & Importance - Lesson
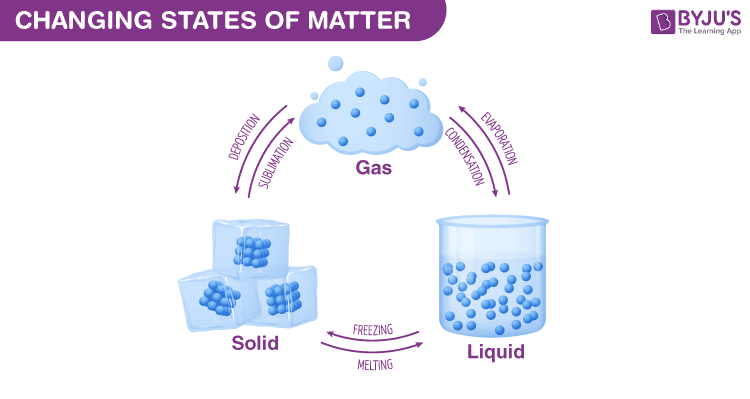
Changing States Of Matter - Solid, Liquid And Gas

Collision Theory- Definition, Explanation, Activation energy
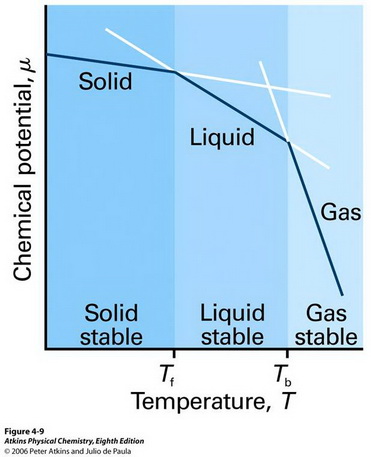
How do the chemical potentials compare for the vapour and liquid

History of climate change science - Wikipedia
What is the significance of the curve part in Z vs. P graph of
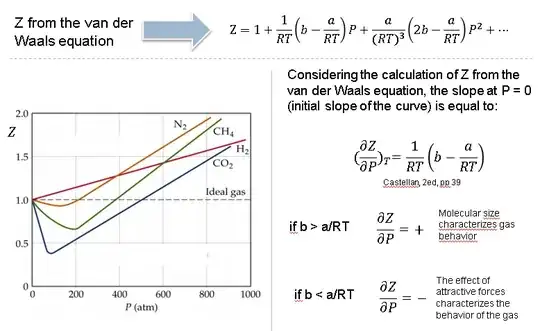
Why do some gases have lower value of Z for a particular pressure
Why do high pressure and low pressure make gases non-ideal? - Quora