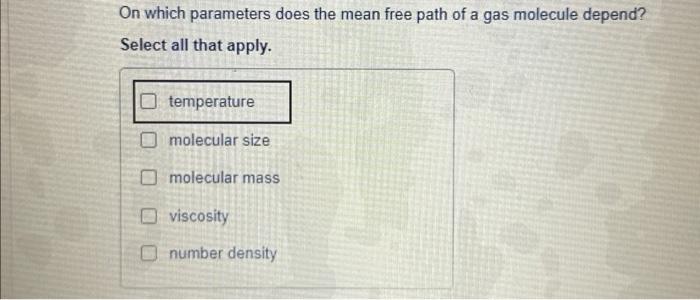

The mean free path of molecules of a gas (radius r) is inversely proportional to

SOLVED: 'The expression for the collision frequency can be written in terms of the parameters of an ideal gas (specifically P and T): Show how this is done and the resultant equation.
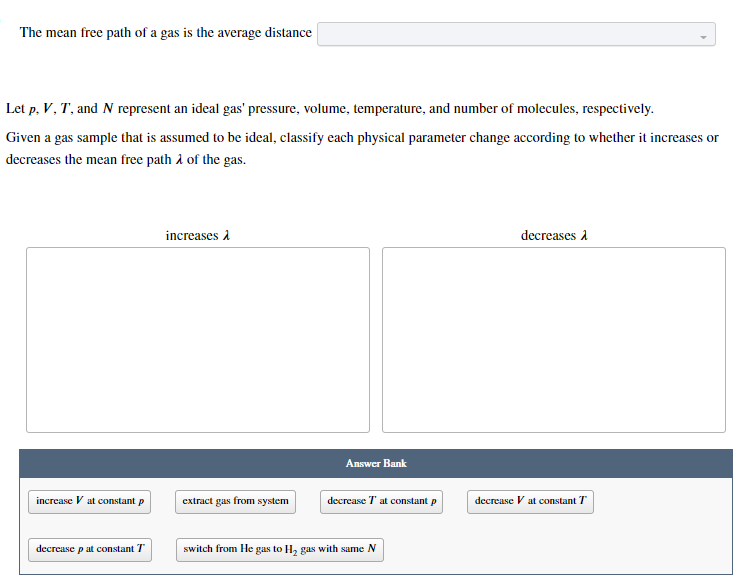
Solved Let p,V,T, and N represent an ideal gas' pressure
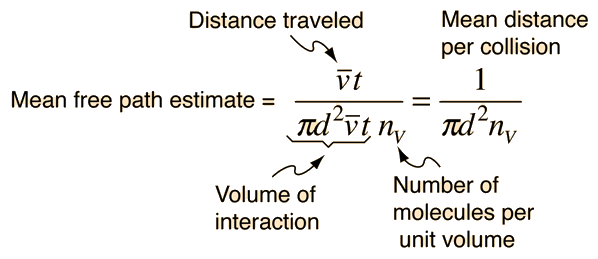
Mean Free Path, Molecular Collisions
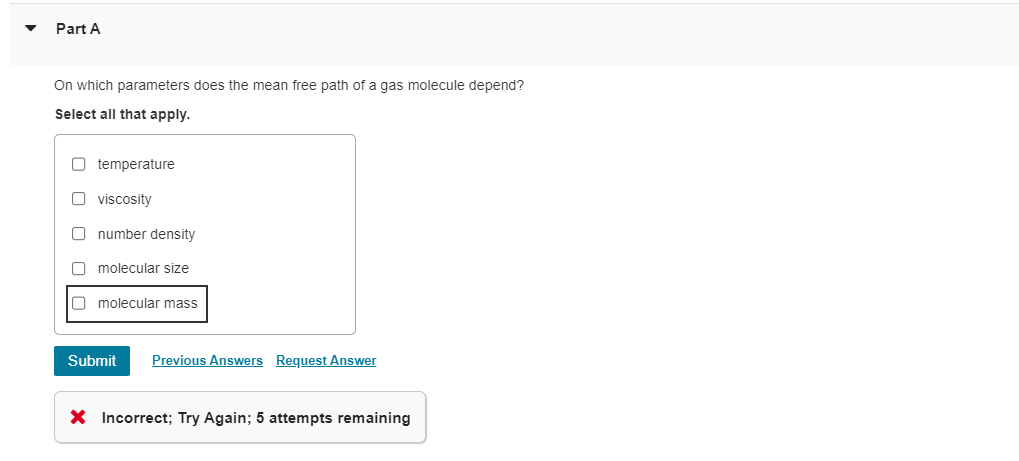
Solved Part AOn which parameters does the mean free path of

Ethiopia Learning - Physics grade 12 page 25 in English
Document Display, NEPIS

What is the Maxwell-Boltzmann distribution? (article)
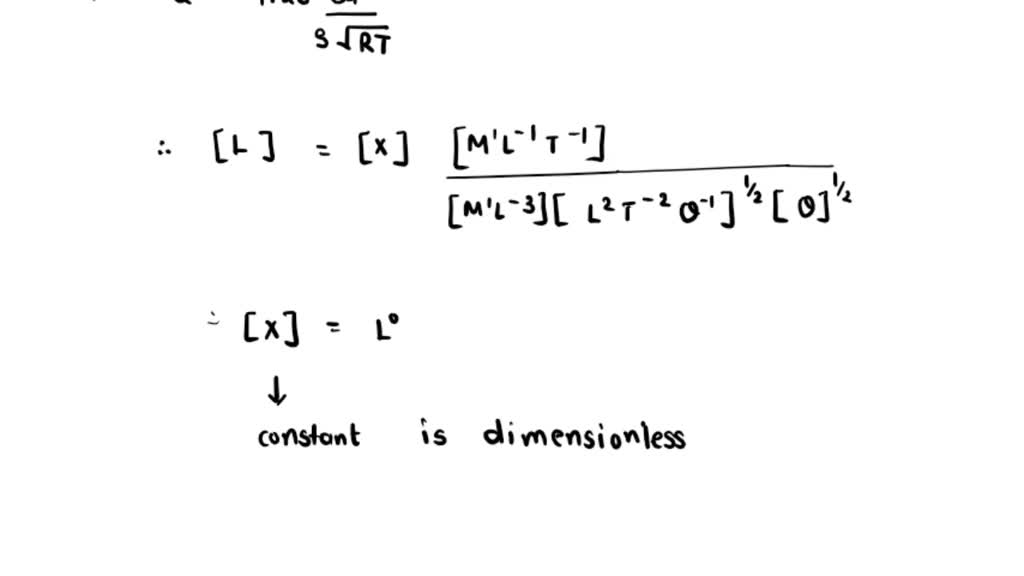
SOLVED: The mean free path of a gas, l, is defined as the average distance traveled by molecules between collisions. A proposed formula for estimating l of an ideal gas is l=1.26 (

Phase transition - Wikipedia
What is the mean free path of an ideal gas? - Quora







