Which of the following statements is/are correct? (a) all real gases are less compressible than ideal gas at high pressures? (6) hydrogen and helium are more co
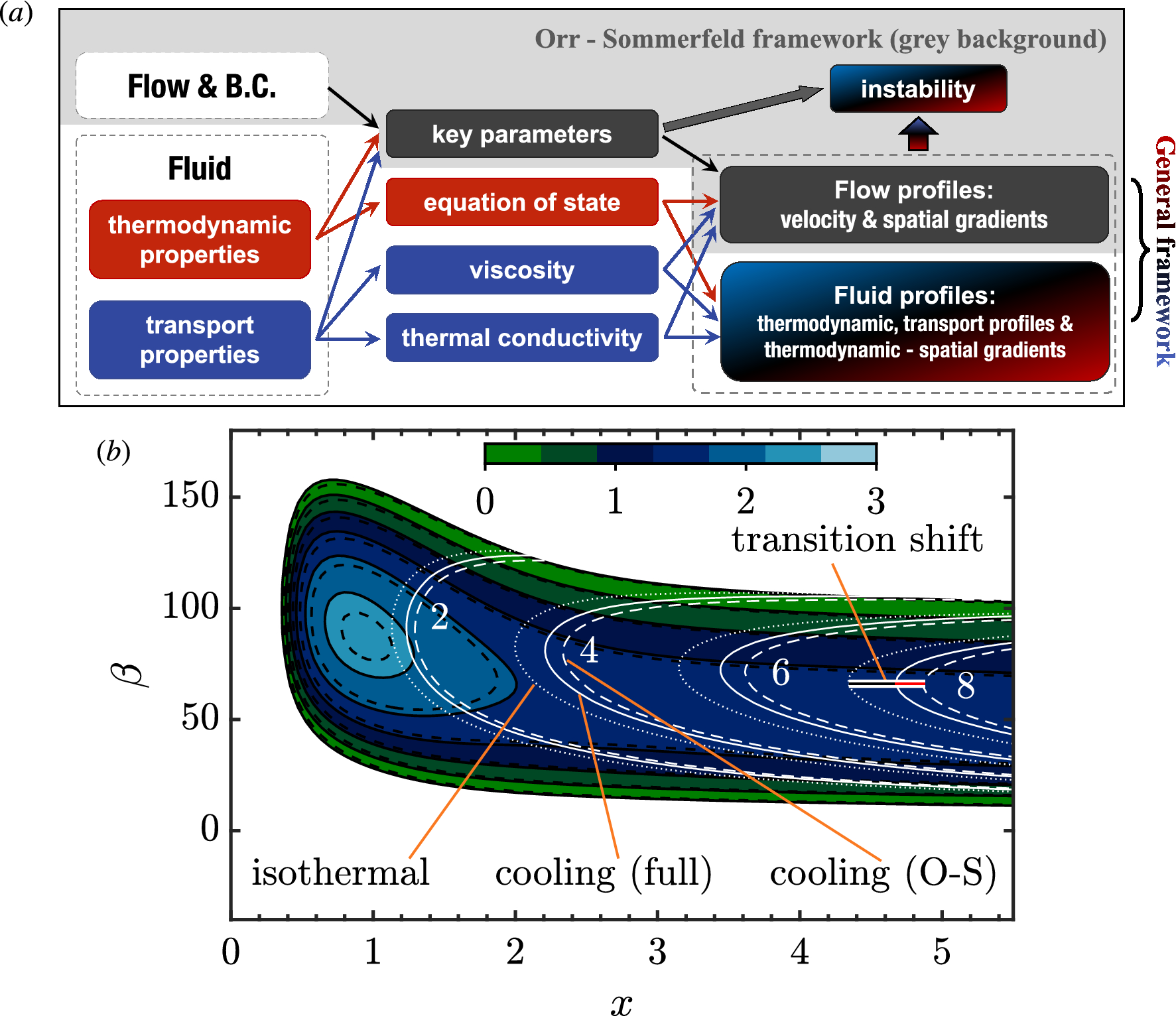
Non-ideal gas behavior matters in hydrodynamic instability
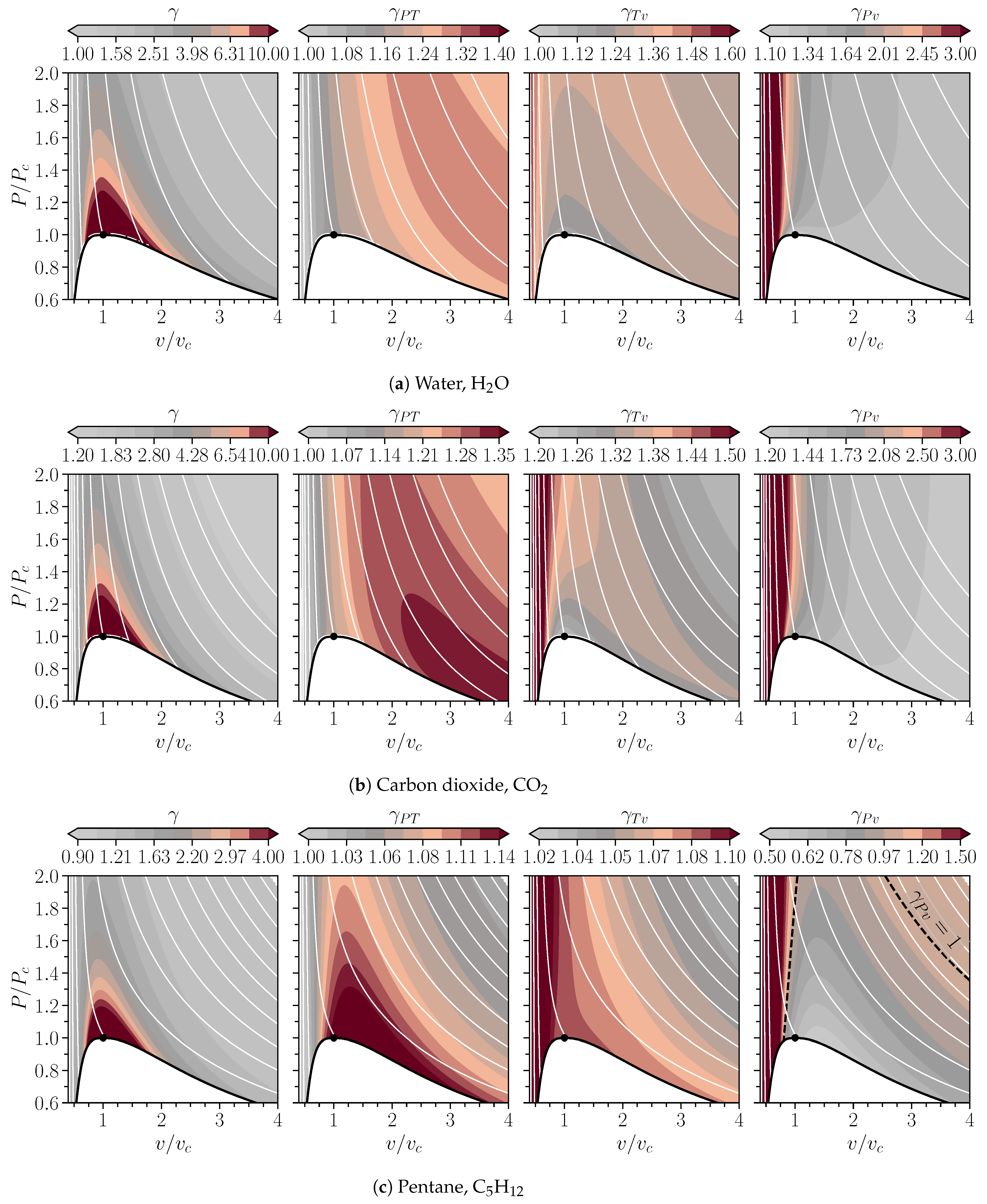
Energies, Free Full-Text

The densities of the three gases O_2 , SO_2 and H_2 are measured 400,K and 1 ,atm . They are 0.8,g/L , dfrac{32}{15} ,g/L and dfrac{1}{25},g/L respectively . Which of the following
Deviation Of Real Gas From Ideal Gas Behavior

Solved Question 13 (3.13 points) Which of the following
The given graph represents the variation of Z (compressibility factor =) versus P, for three real gases A, B and C. Identify the only incorrect statement. [JEE 2006]a)For the gas A, a =

Correlation for natural gas heat capacity developed
Which gases behave least like ideal gases? - Quora

Which of the following statements is/are correct? (a) all real gases are less compressible
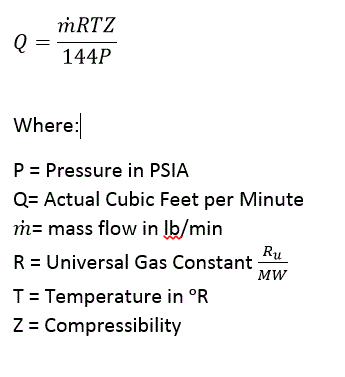
Volume and Mass Flow Calculations for Gases
We say that due to repulsive force present in real gases,volume occupied by the molecule is more than ideal gas that is,correction factor is given by (V nb)but if we consider attractive
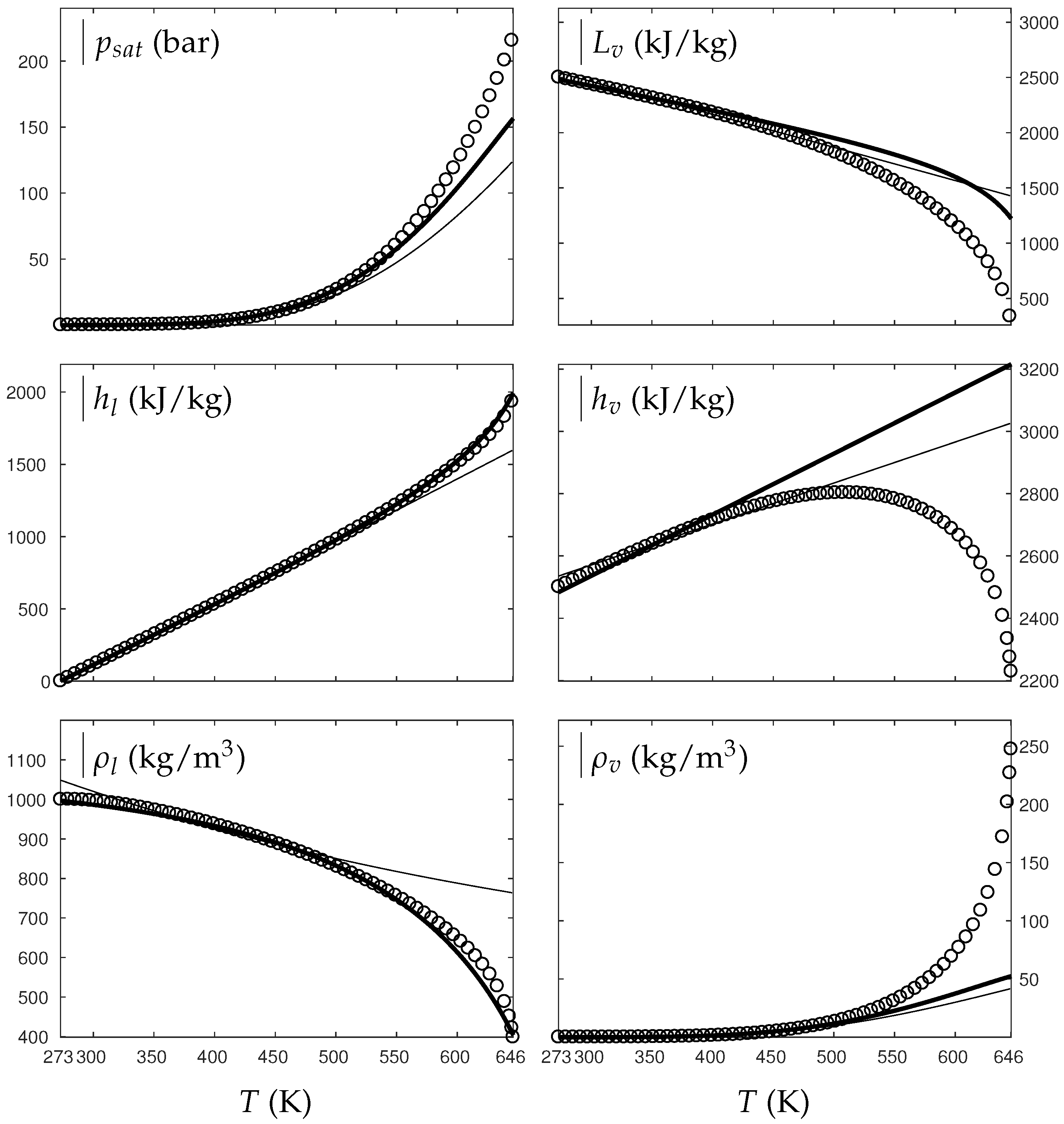
Fluids, Free Full-Text

A novel numerical approach for simulating low-pressure and high-pressure non-equilibrium condensation in real gases - ScienceDirect
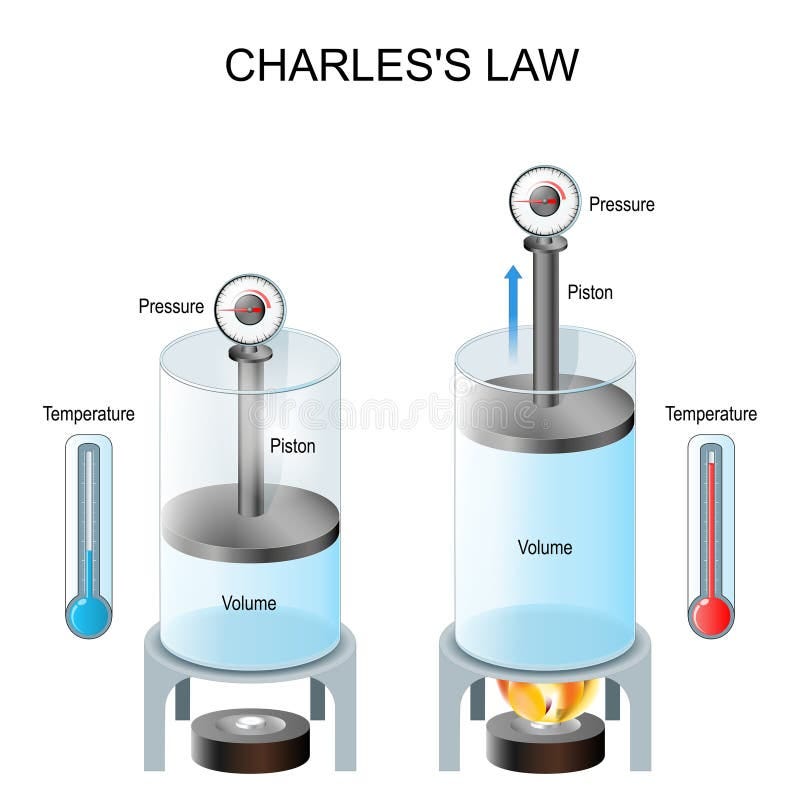
Charles law: volume and temperature

Gas - Behaviour, Properties, Physics