
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5

18. The compressibility factor one mole of a vanderwaal's gas 0°C and 100 atm pressure is found to be 0.5. Assume that the volume of gas molecule is negligible calculate the vanderwaals

Pb 00 atmosphere 10. (JEE 2001 The compression factor (compressibility factor) ression factor (compressibility factor) one mole of a vander Waals gas 0°C and 100 atmos pressure is found to be 0.5.

The Properties of Gases: Real Gases (1C) Flashcards
The compression factor (compressibility factor) for one mole of a van der..
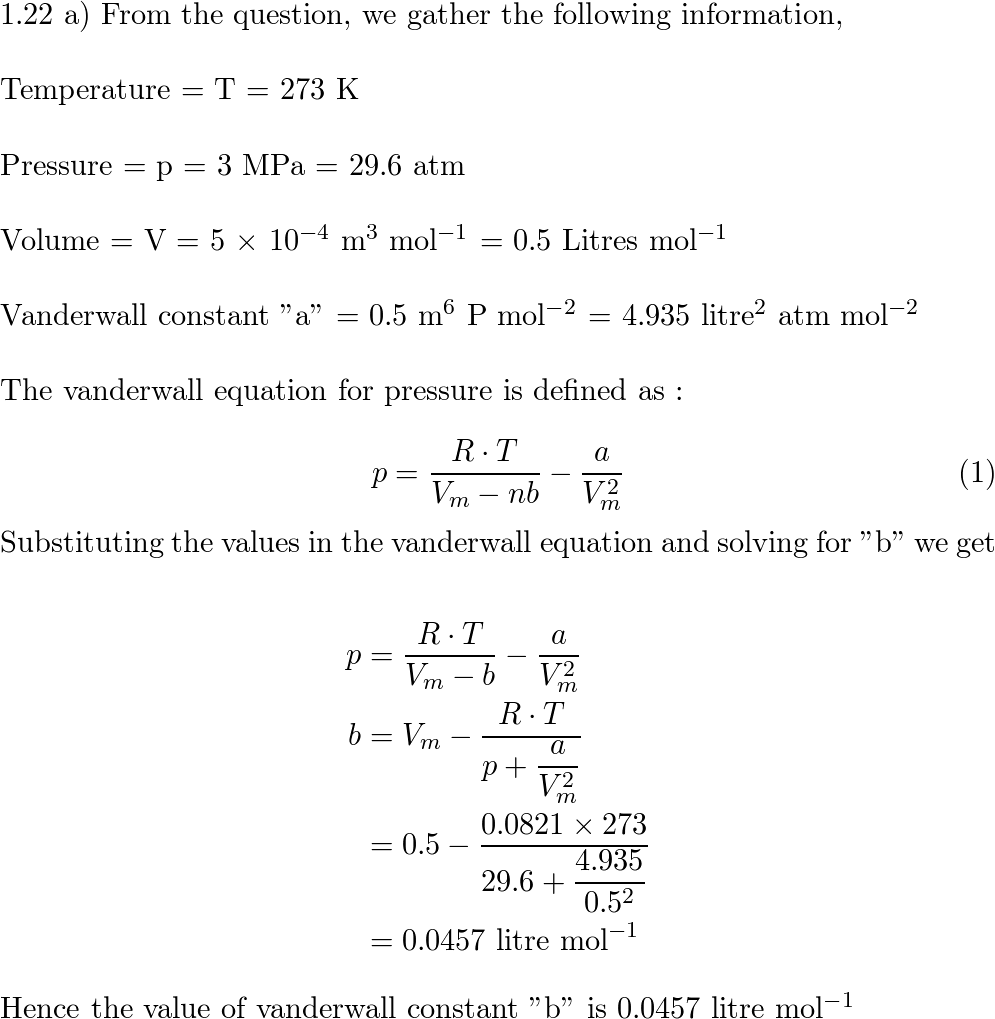
a) A certain gas obeys the van der Waals equation with $a =
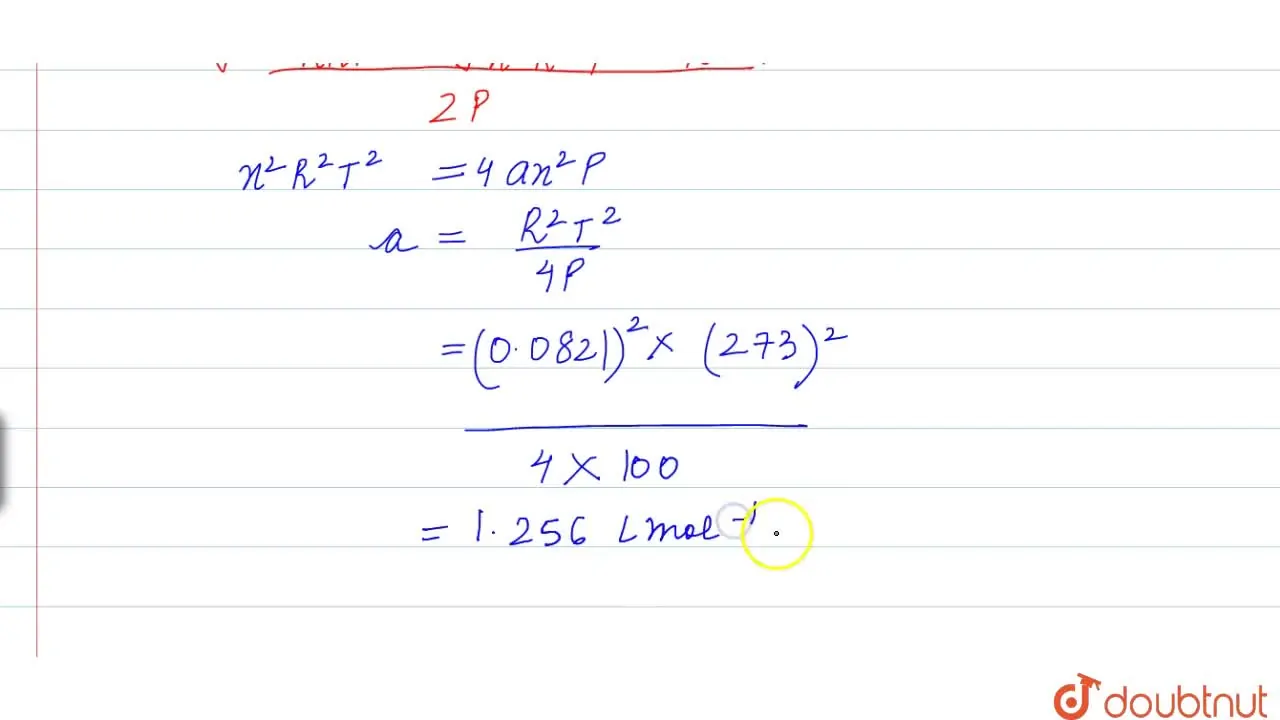
The compressibility factor for definite amount of van der Waals' gas a

Bengali] The compressibility factor (Z) of one mole of a van der Waal
The value of compression factor at the critical state of a vander waals gas is
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas
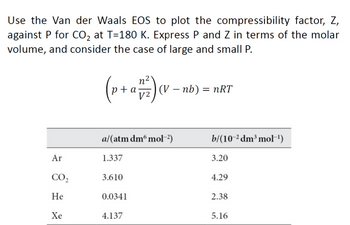
Answered: Use the Van der Waals EOS to plot the…

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
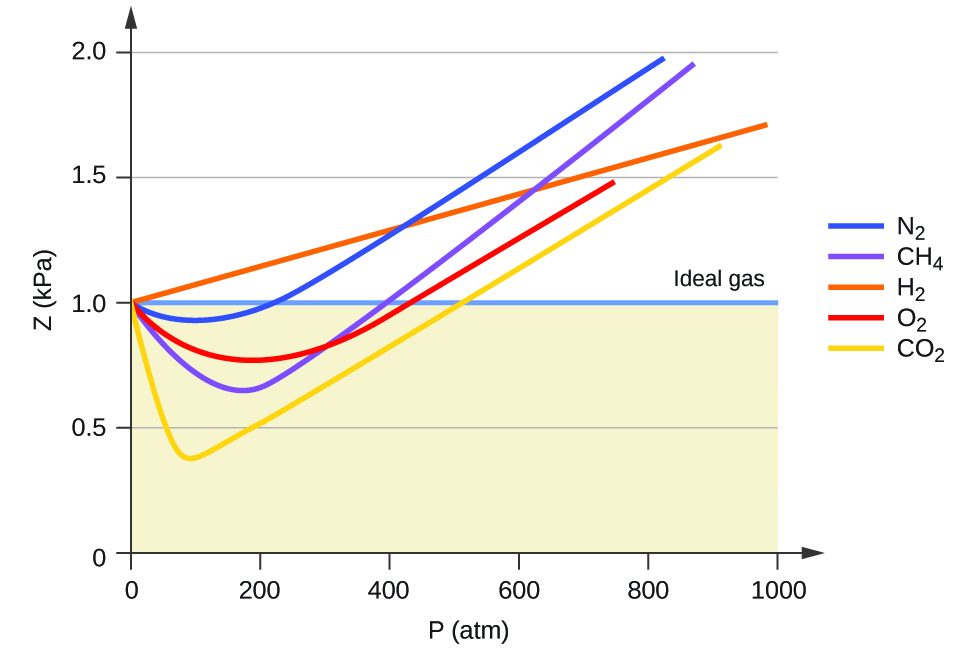
8.6 Non-Ideal Gas Behavior – General Chemistry 1 & 2







