
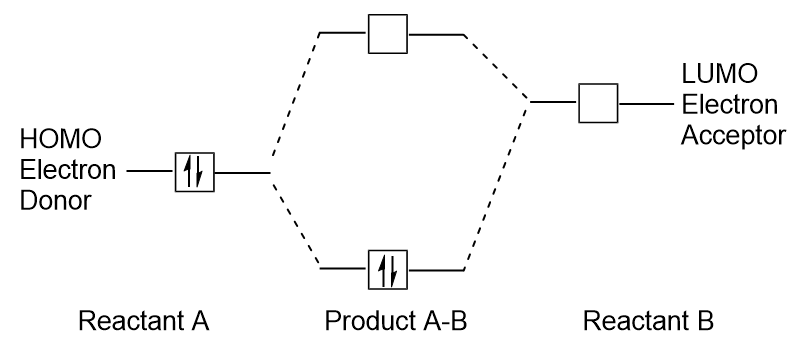
Consider: The description of this image in my textbook is as follows: In order to form a bond, the HOMO (the highest occupied molecular orbital) of one species must interact with the LUMO (the lo

The Spectroscopy of Nitrogenases

Non-covalent interactions from a Quantum Chemical Topology perspective
Molecular orbital theory & predicting the stability of a molecule? - Chemistry Stack Exchange

Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry. - Abstract - Europe PMC
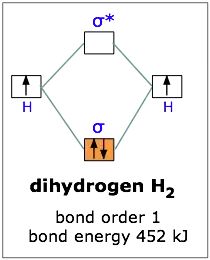
Molecular Orbitals – Introductory Chemistry – 1st Canadian Edition

Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds - ScienceDirect
In Molecular Orbital Theory, why do sigma and sigma* bonding and antibonding orbitals have a greater difference of energy than pi and pi* orbitals? - Quora

Organic Chemistry - Resonance and Molecular Orbital Diagrams : r/Handwriting

organic chemistry - Orbital Interaction for electrophile and nucleophile - Chemistry Stack Exchange

organic chemistry - When is donation into an anti-bonding MO stabilising? - Chemistry Stack Exchange

A Bonding Quandary—or—A Demonstration of the Fact That Scientists Are Not Born With Logic - Alvarez - 2009 - Chemistry – A European Journal - Wiley Online Library
In Molecular Orbital Theory, why do sigma and sigma* bonding and antibonding orbitals have a greater difference of energy than pi and pi* orbitals? - Quora
/cdn.vox-cdn.com/uploads/chorus_image/image/70403442/19BR_TiltedTowers.0.jpg)






