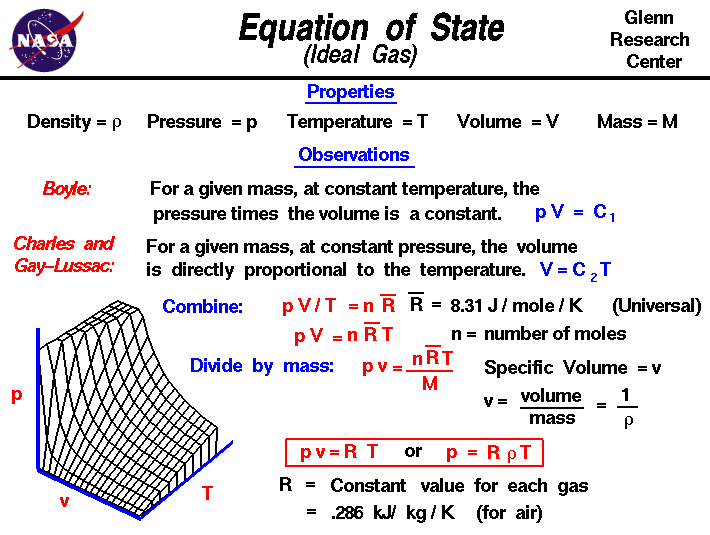
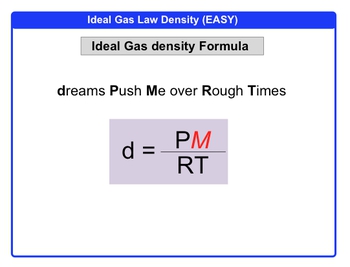
I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is
The World Is Wasting Our Irreplaceable Helium, And Nobody Cares : r/Physics
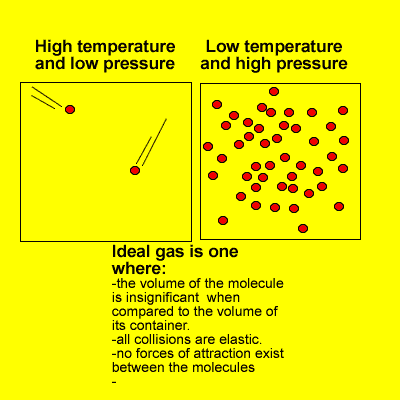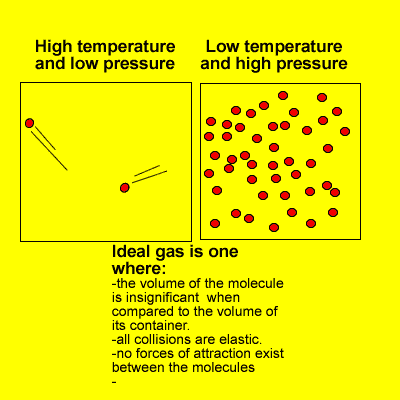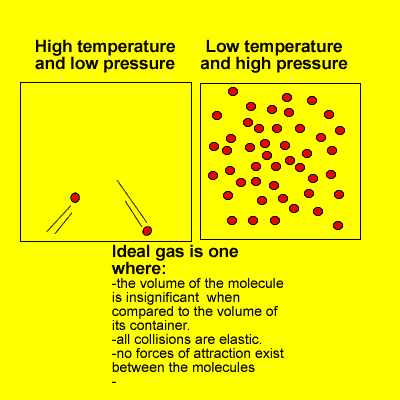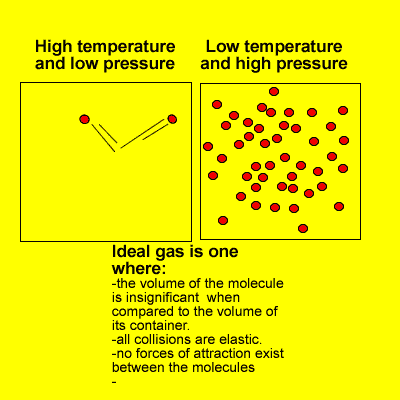
Chemistry - Ideal gas-kinetic theory of gases

PPT - Balloons and Gas Laws PowerPoint Presentation, free download - ID:9720799

Equipartition theorem - Wikipedia
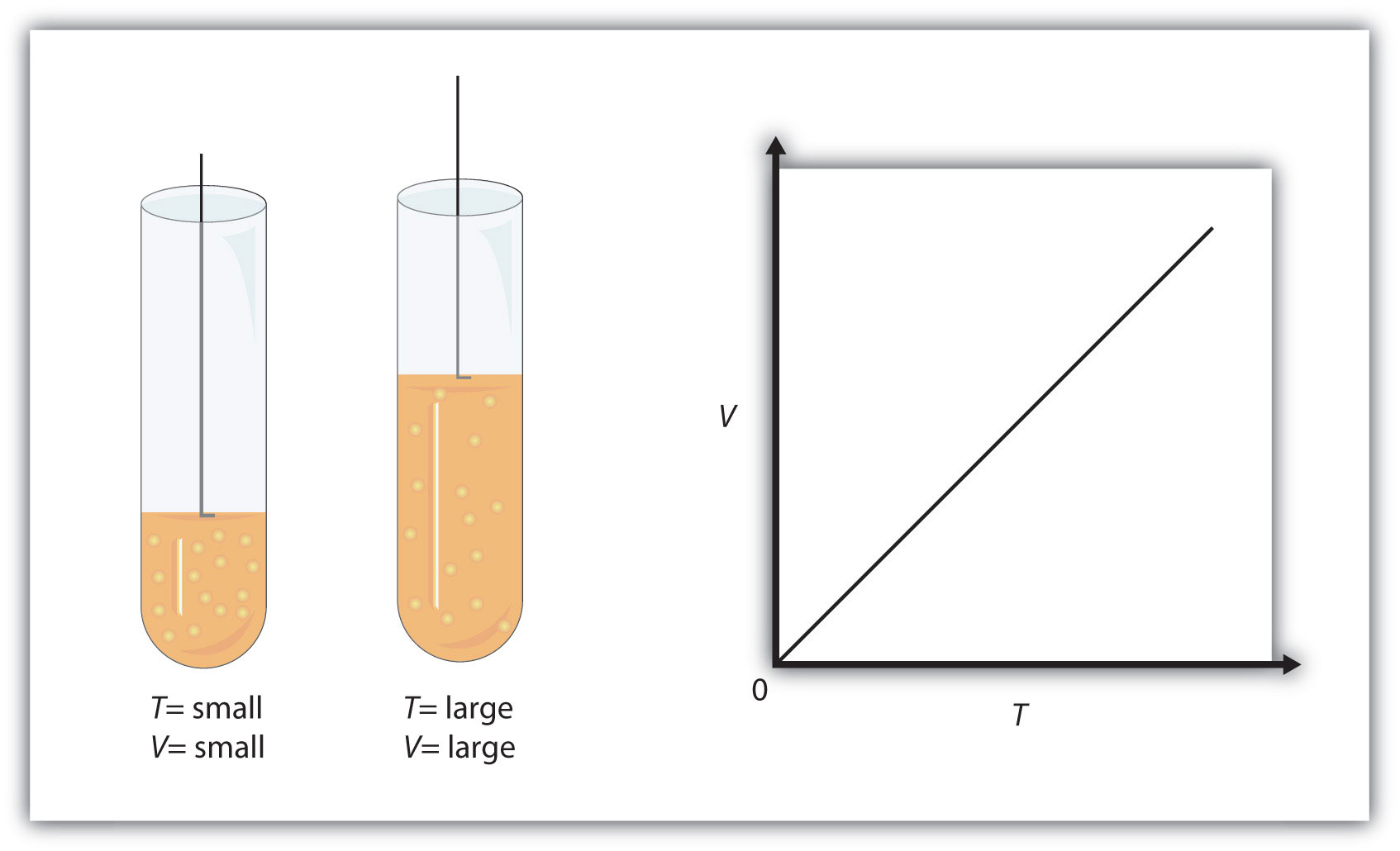
Chapter 9 - Gases - CHE 110 - Introduction to Chemistry - Textbook - LibGuides at Hostos Community College Library

Ideal gas law - Wikiversity

Why is HCl less ideal than CH4, He and N2? - Quora

10.4: The Ideal Gas Equation - Chemistry LibreTexts

Kinetic Theory of Gases, PDF, Gases
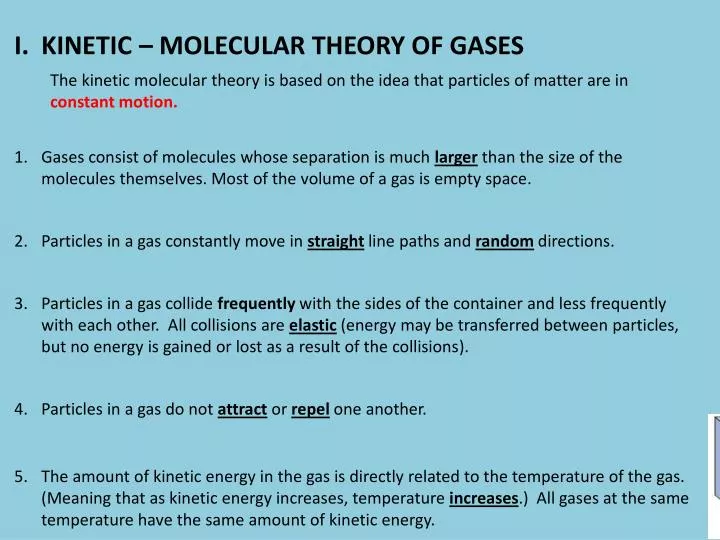
PPT - KINETIC – MOLECULAR THEORY OF GASES PowerPoint Presentation, free download - ID:4176699
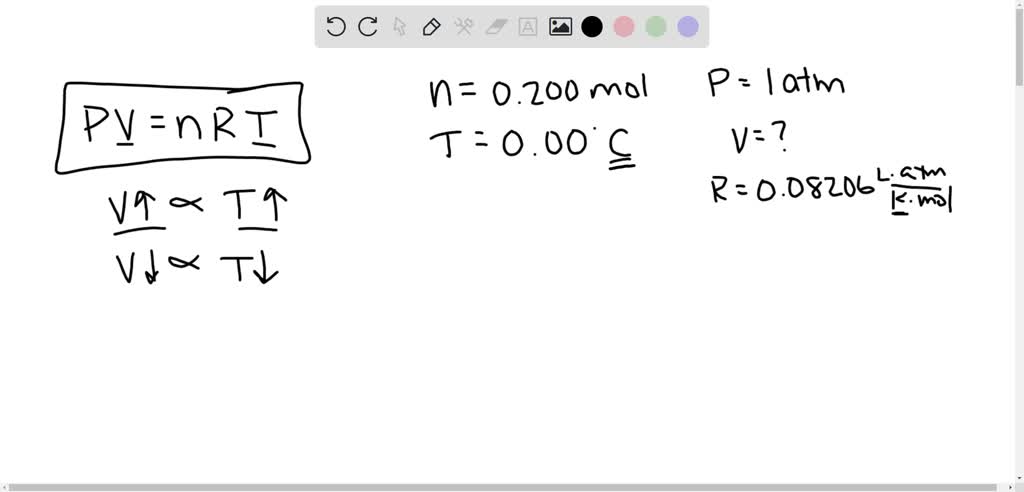
SOLVED: The ideal gas law predicts gas behavior including the relationships between the number of moles, volume, pressure, and temperature. Predict the changes in volume of a helium-filled balloon at different temperatures.