(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

At 300 K, the density of a certain gaseous molecule at 2 bar is double

Solved We begin by showing that the compressibility factor
Why does CH4 have a greater value of van der Waals' constant than

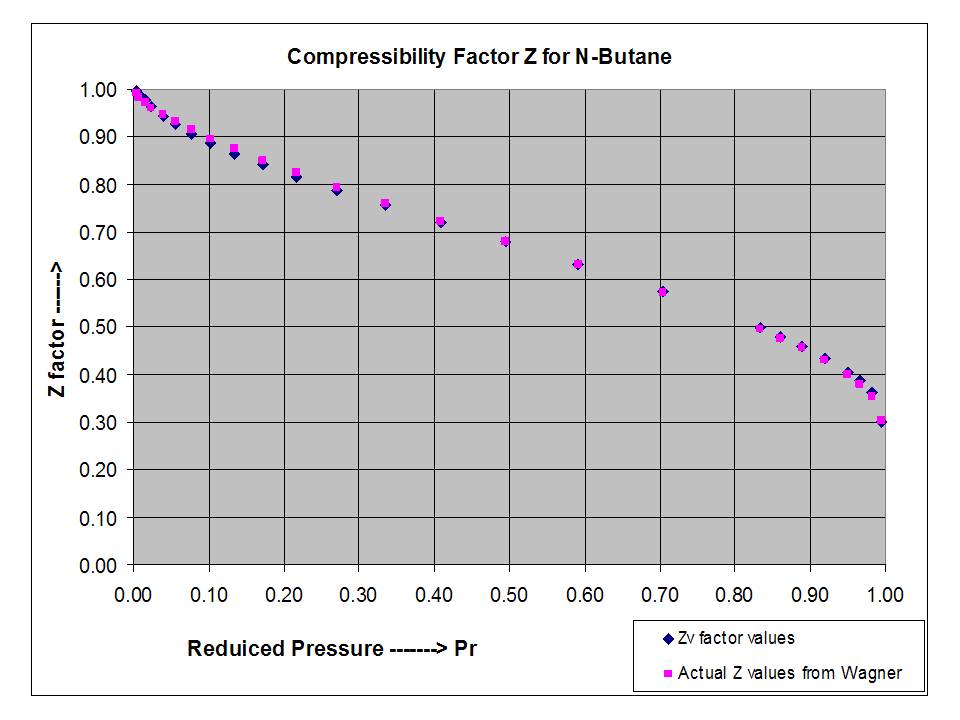
Investigation of the Properties of Hydrocarbon Natural Gases Under

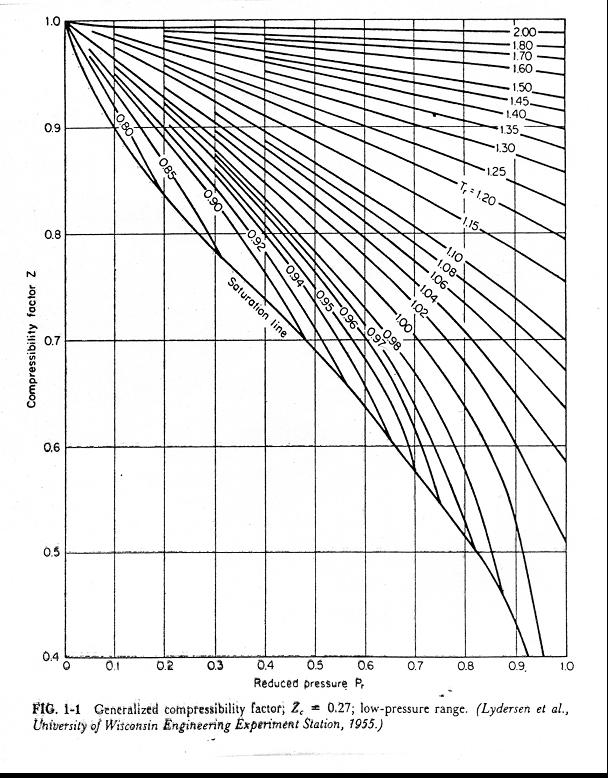
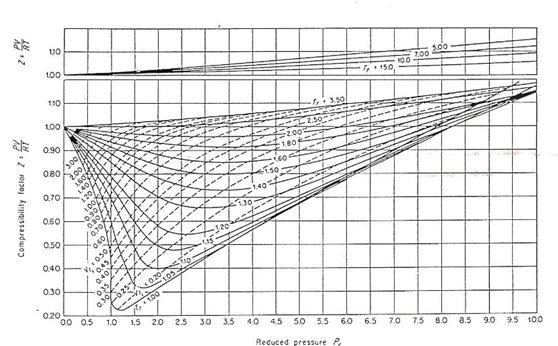
At 273K temp, and 9 atm pressure, the compressibility fog a gas is 0.9

Solved Data: Ideal gas constant R 8.314 J mol1 K1 1 (a)
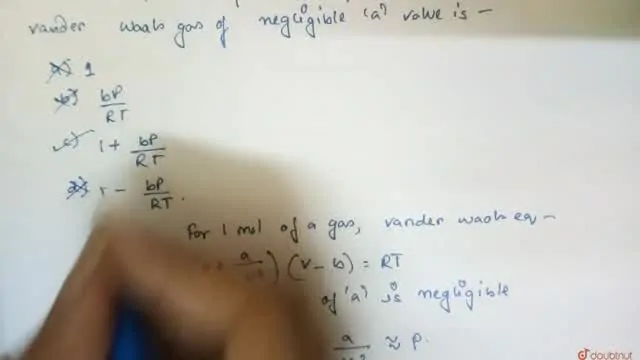
Bengali] The compresibility factor (Z) of one mole of a van der waals

jo 22] What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible.
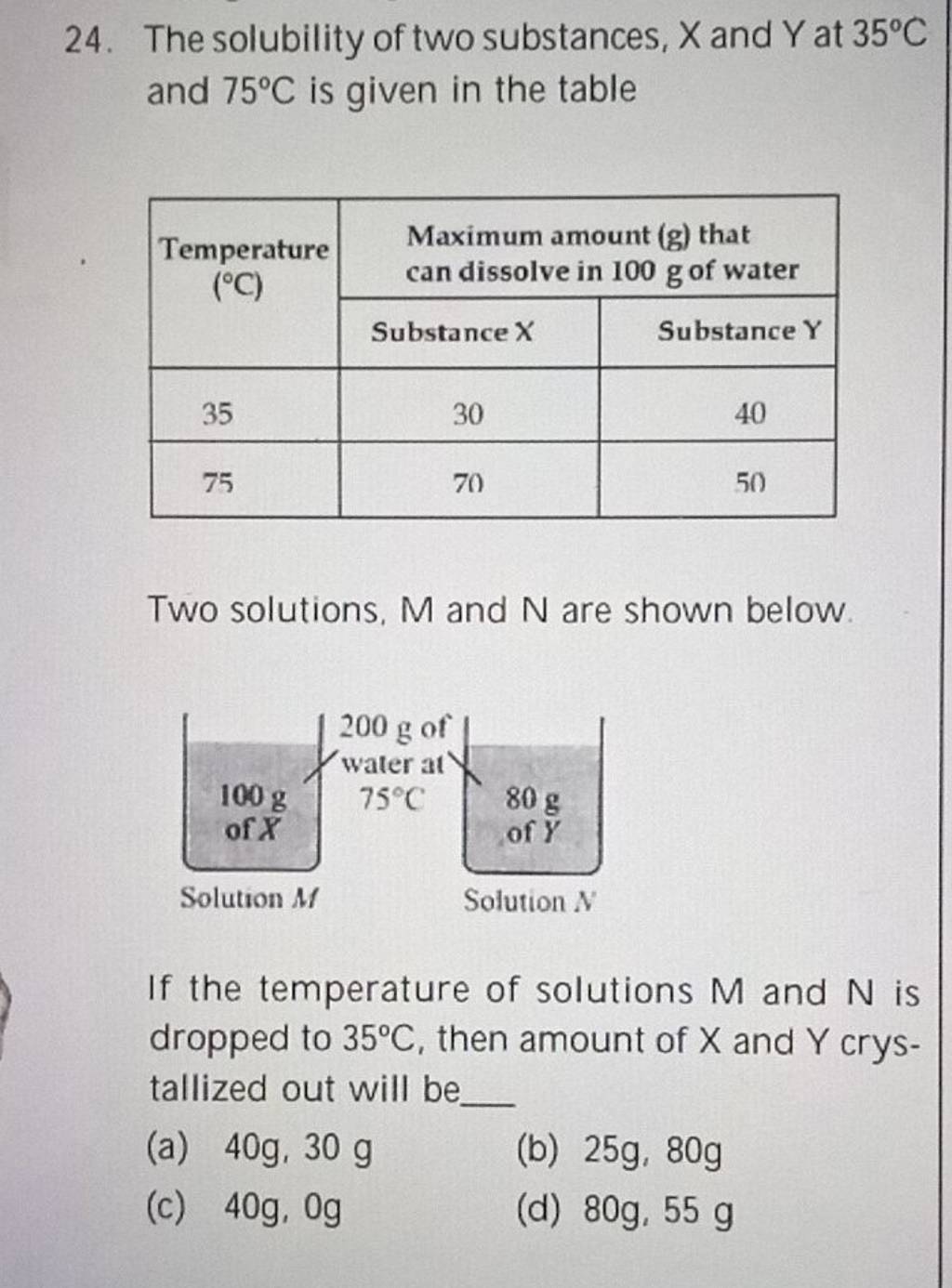
The solubility of two substances, X and Y at 35∘C and 75∘C is given in th..

Van Der Waals Equation - an overview