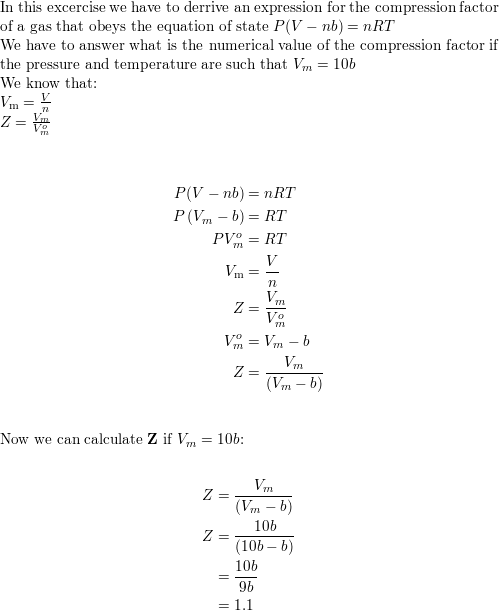Compressibility Factor of Gas Overview, Equation & Chart
What is the value of compressibility factor in terms of vander

The compressibility factor of a gas is defined as Z=PV/nRT. The

What is the compressibility factor? What is its value an ideal gas
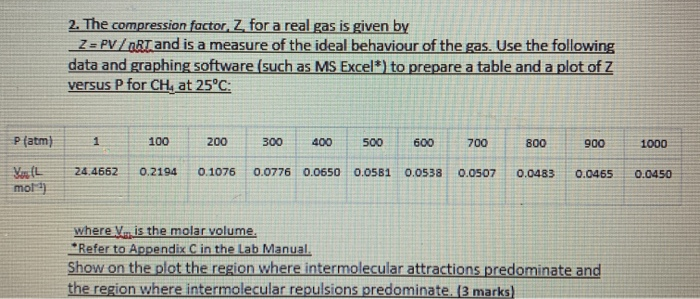
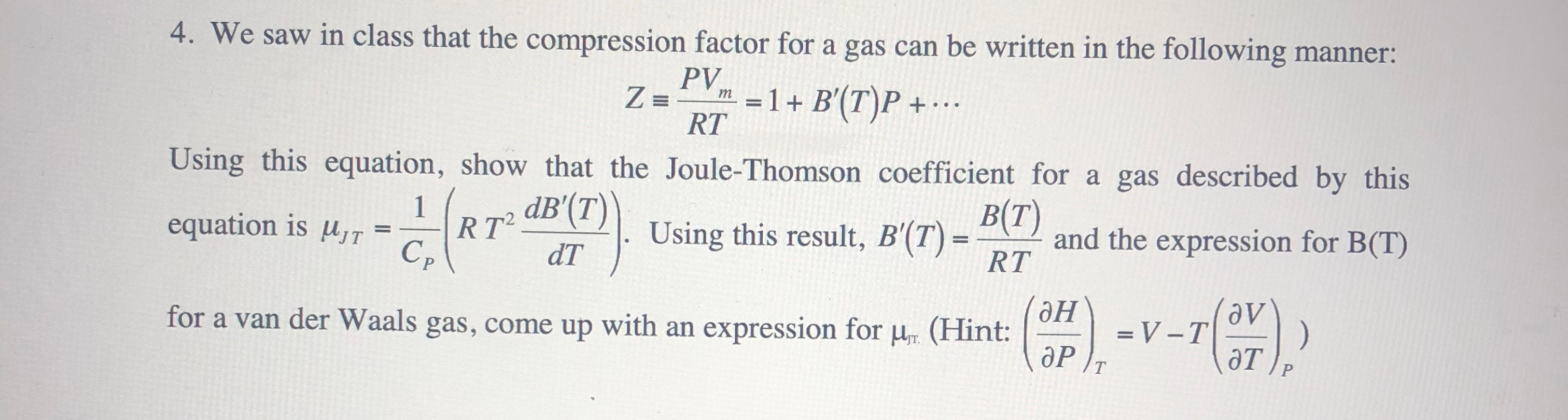
Solved 2. The compression factor, Z. for a real gas is given

Explain how the compression factor varies with pressure and

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
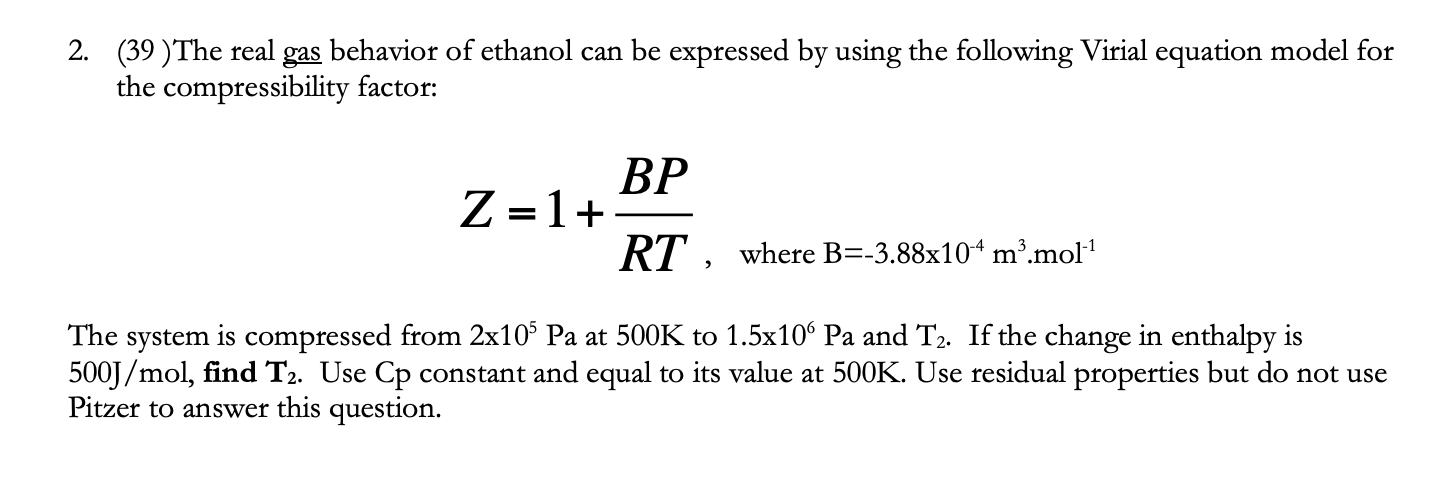
Solved The real gas behavior of ethanol can be expressed by

The compressibility factor of an ideal gas isa)0b)1c)2d)4Correct

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

The given graph represents the variation of compressibility factor

Real gasses For an ideal gas, the compressibility factor Z = PV

Bengali] The compressibility factor (Z) of one mole of a van der Waal

physical chemistry - Compressibility Factor Graph - Which gas