Answer to Solved What is the equilibrium constant (Kp) at 45 °C for
✓ Solved: At 2200^∘ C, Kp=0.050for the reaction N2(g)+O2(g) ⇌ 2 NO(g) What is the partial pressure of

The equilibrium constant (K(p)) at 27^(@)C for a homegenous gasesous r

Select the correct statement(s) abouf equilibrium constant (i) In standa..

Equilibria: Calculating the equilibrium constant Kp. Qu. 2 of 2
A complex equilibrium question

For the reaction, A(g) + B(g)rightarrow C(g) + D(g), Delta H^o and Delta S^o are, respectively, -29.8 kJ mol^{-1} and -0.100 kJ K^{-1} mo1^{-1} 298 K. The equilibrium constant the reaction 298

45 Calculate the equilibrium constant the following reaction 298 K and 1 atm pressure. C (graphite) + H2O(l) → CO(g) + H2(g) Given : Af H°, [H2O (1)] = - 286.0 kJ
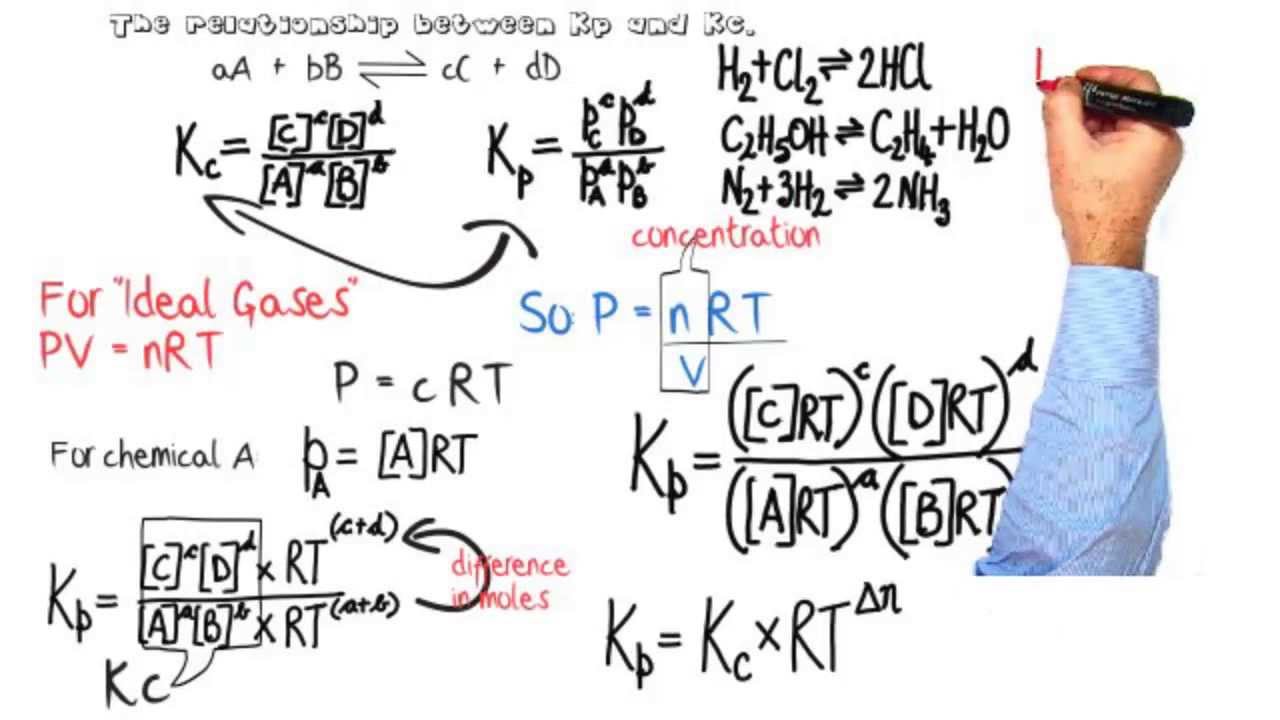
Equilibria: Relationship between equilibrium constants Kp & Kc.

Answered: Which of the following is the correct…

Solved Consider the equilibrium and answer the questions

Ethiopia Learning - Chemistry grade 11 page 275 in English
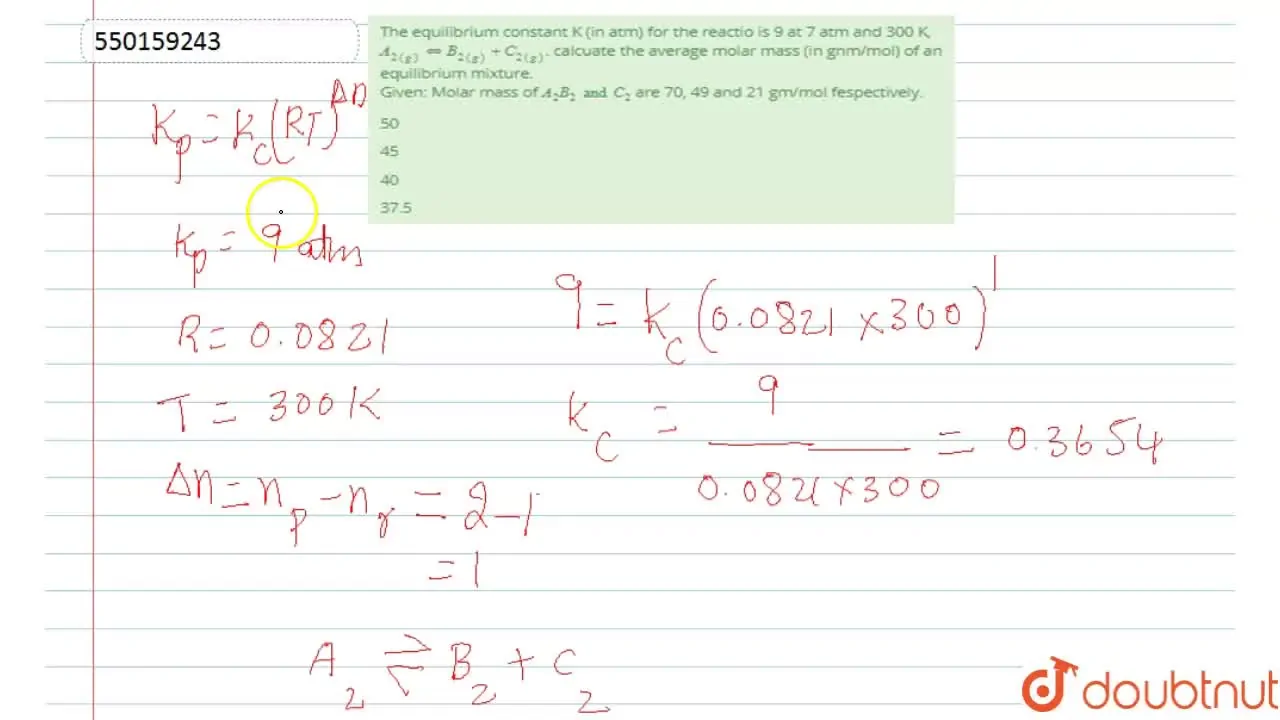
Telugu] The equilibrium constant K (in atm) for the reactio is 9 at 7