In this article, you will learn about the steady state approximation, an important tool in understanding the kinetics of consecutive reactions.

i.ytimg.com/vi/a1f8gsl9908/hq720.jpg?sqp=-oaymwE7C
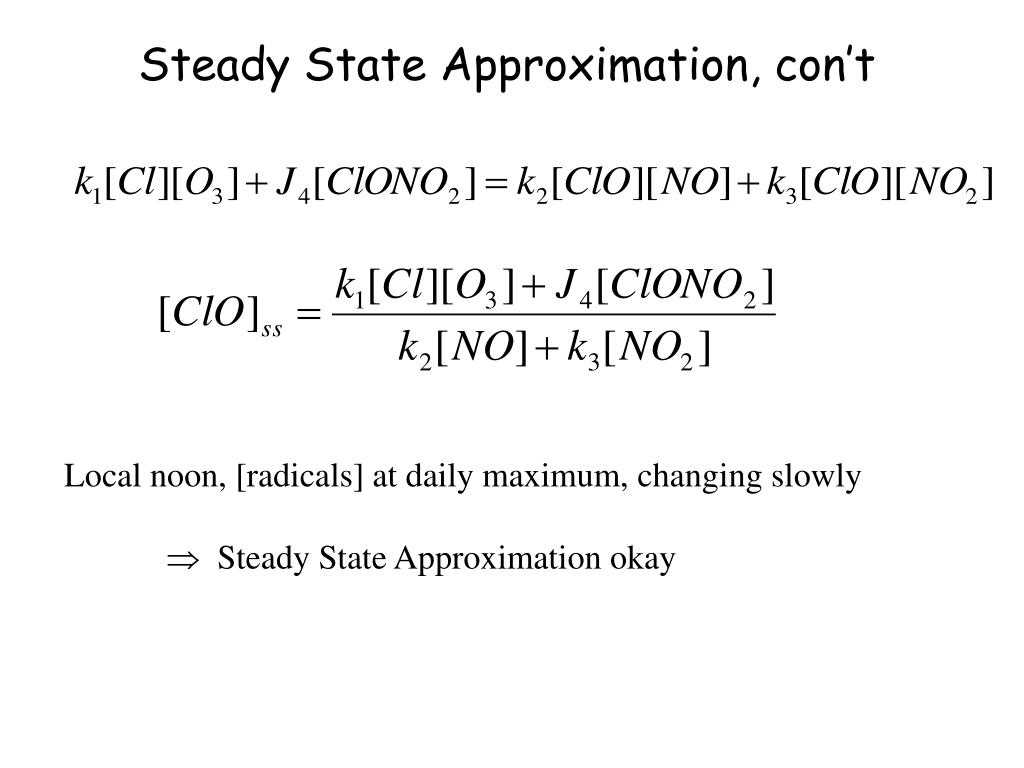
PPT - IV. Kinetics Introduction (Pseudo) First Order Approx. Steady State Approximation PowerPoint Presentation - ID:138905
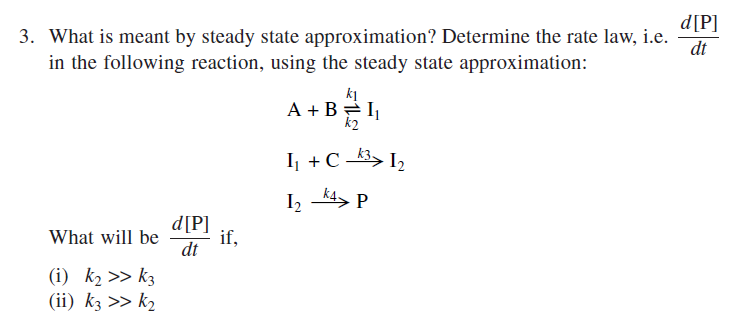
Solved What is meant by steady state approximation?

10.2 Approximate treatment of rate equation - ppt download

Chemical kinetics - The Arrhenius equation, Reaction mechanisms, Steady-state approximation and Kinetics vs. Thermodynamic control Flashcards

Chemical kinetics: accounting for the rate laws - ppt video online download
Lecture 21. The Steady State Approximation. - TIB AV-Portal
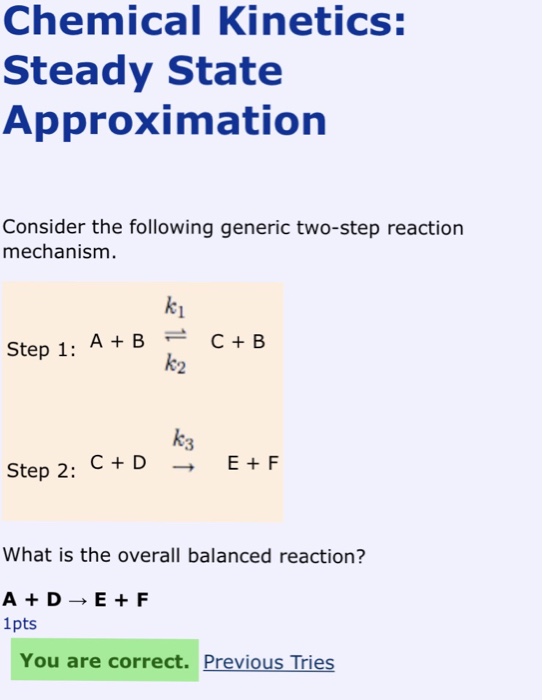
Solved Chemical Kinetics: Steady State Approximation
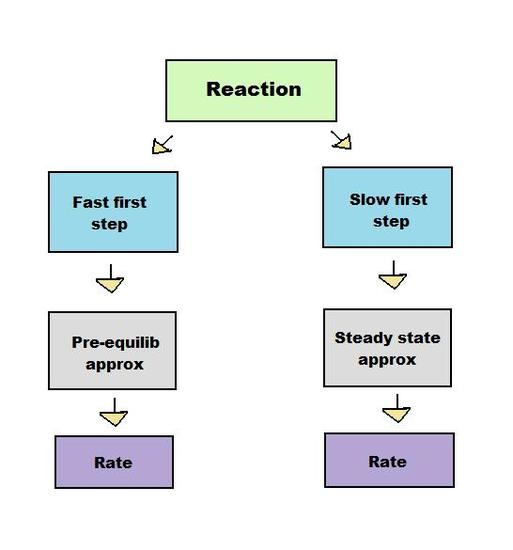
3.2.2: Pre-equilibrium Approximation - Chemistry LibreTexts
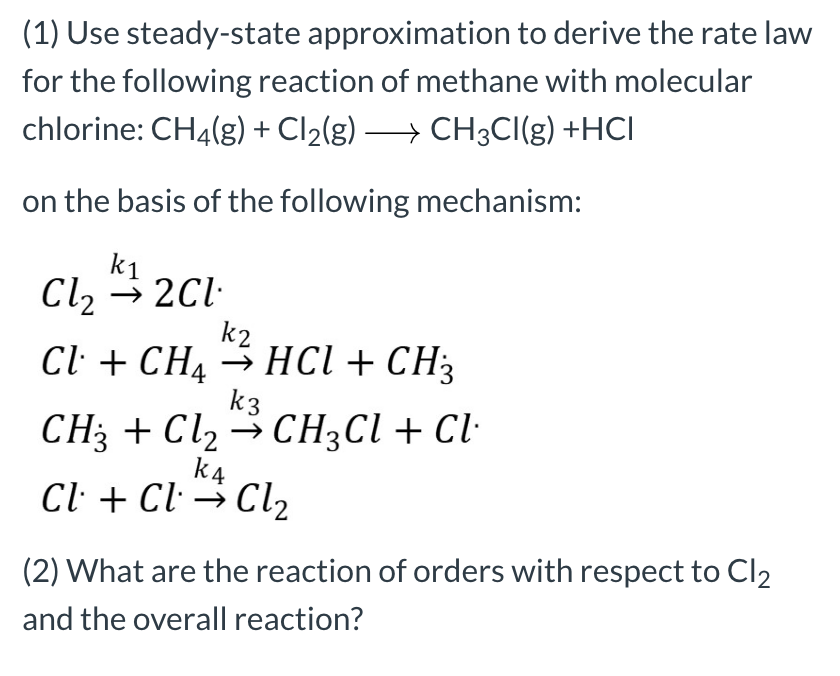
Solved (1) Use steady-state approximation to derive the rate

5.9 - Steady-State Approximation

SOLVED: 5. Use the steady-state approximation method to determine the rate law for the given reaction: 2A,B>4AB+B The three-step mechanism is given as follows: AB+AB>AB+AB+B AB+AB>2AB

SOLVED: Consider the following reaction: A + 2B â†' E. Using the steady-state approximation, determine which of the following can determine the overall rate law (assuming that the rate-determining step is unknown)

Overall Reaction Rate using Steady-State Approximation – Detailed Solution

5 The steady state approximation. Simulations are shown for a simple