
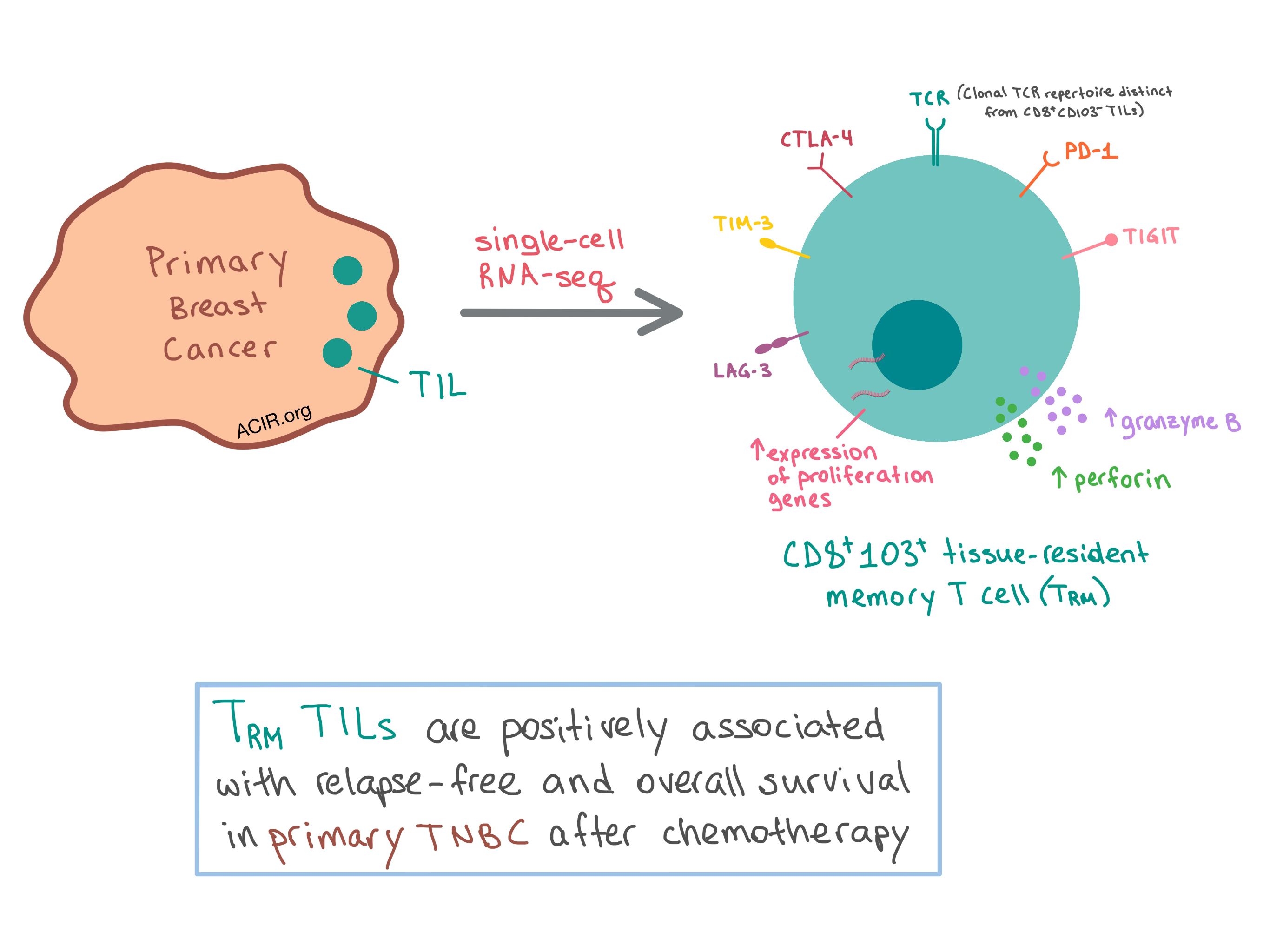
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.
Sacituzumab Earns Regular FDA Approval For TNBC NCI

Sacituzumab Earns Regular FDA Approval For TNBC NCI
.jpg)
New FDA Alert Warns of Drug Combination for Advanced Triple-Negative Breast Cancer Patients

Biomolecules, Free Full-Text
Trodelvy for the Treatment of Advanced Triple-Negative Breast Cancer
ASCO Experts Update Guidance for Sacituzumab Govitecan (Trodelvy

Sacituzumab govitecan in previously treated hormone receptor

Sacituzumab Govitecan - an overview

Michael Weingarten (@NCISBIRdirector) / X
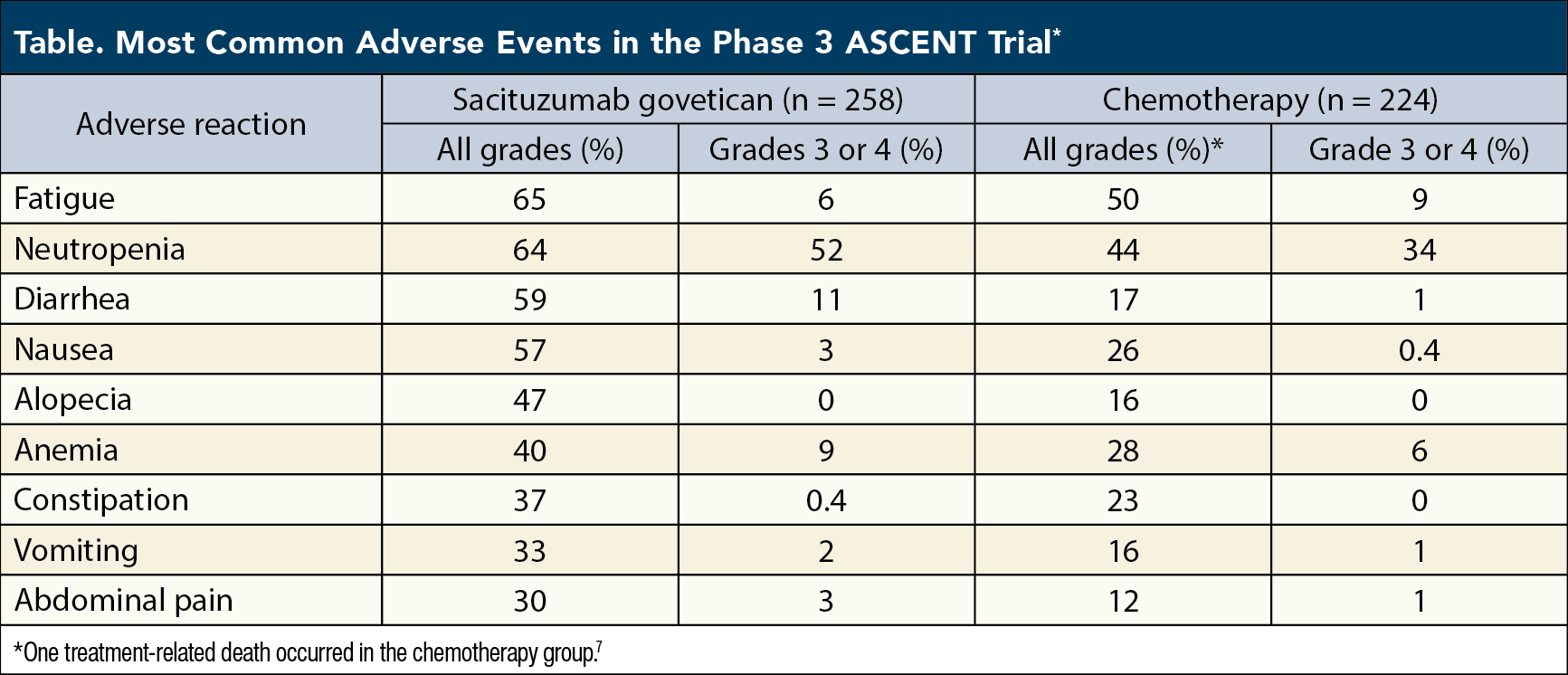
Sacituzumab Govitecan Moves to Second-Line Therapy for Metastatic

Three Treatment Modalities Emerge for Patients with Triple

Recent advances in targeted strategies for triple-negative breast cancer, Journal of Hematology & Oncology

FDA Approves Sacituzumab Govitecan for Triple-Negative Breast

Recent advances in targeted strategies for triple-negative breast cancer, Journal of Hematology & Oncology
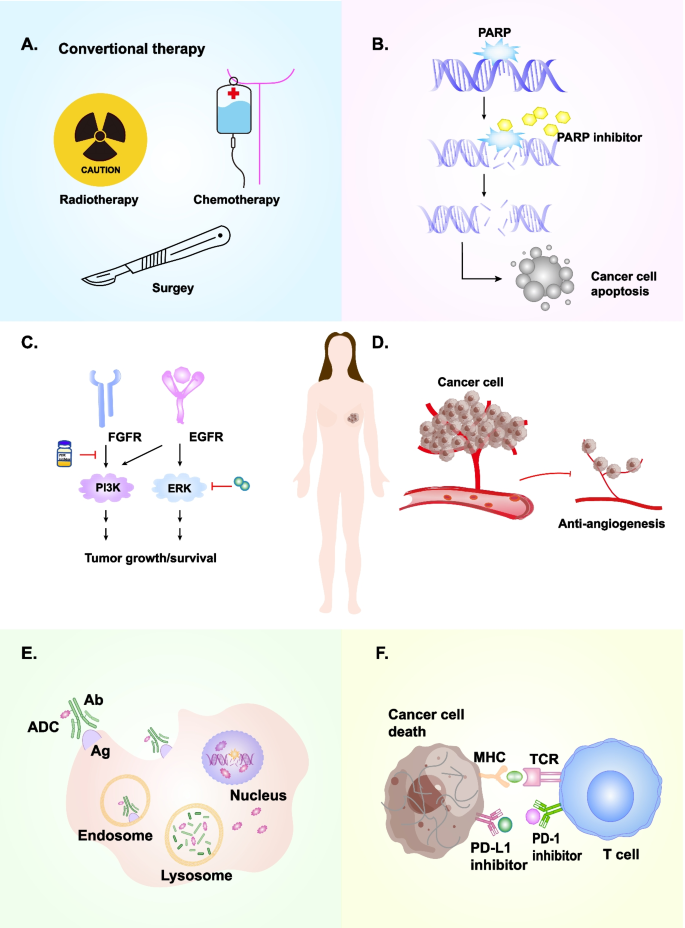
Recent advances in targeted strategies for triple-negative breast


)




/cdn.vox-cdn.com/uploads/chorus_image/image/59342725/D76E413A_DF64_4280_8491_00E6C134D427.156.jpeg)