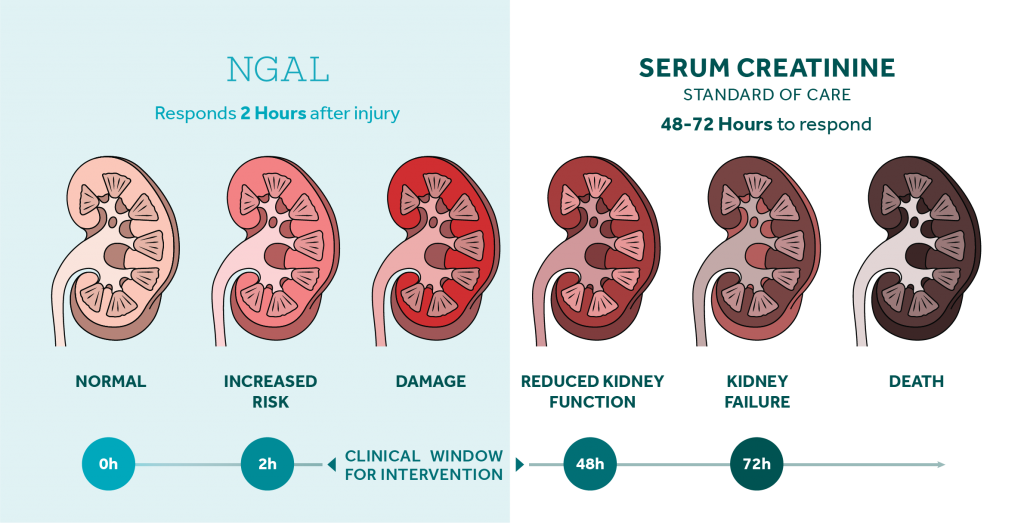
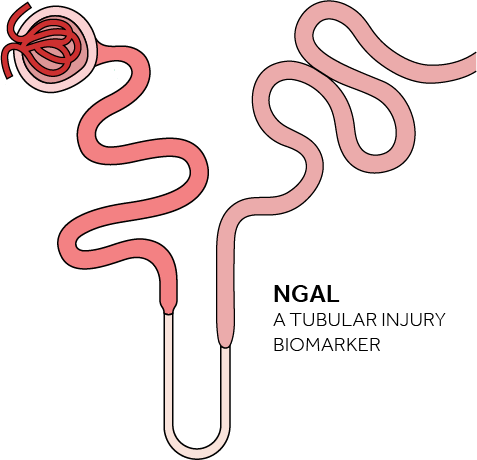
The NGAL Test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine and plasma on automated clinical chemistry analyzers. NGAL measurements are useful in the risk assesment of AKI.
NGAL - Bioporto
/wp-content/uploads/2019/11/Antibody.j

BioPorto Diagnostics A/S: Contact Details and Business Profile

Cutoff values for predicting congestive acute kidney injury.

PDF) Does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) & serum creatinine (sCr) for acute kidney injury (AKI) diagnosis
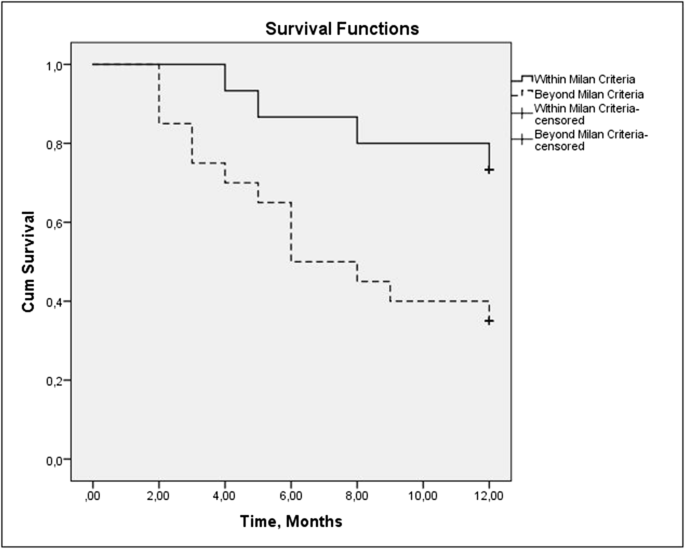
May Neutrophil Gelatinase-Associated Lipocalin (NGAL) Level Predict Mortality in Patients with Hepatocellular Carcinoma (HCC)?

PDF) Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury

NGAL - Bioporto

Home - Bioporto US
Point-of-care neutrophil gelatinase-associated lipocalin (NGAL) tests — NIHR Community Healthcare MIC

First Acute Kidney Injury Diagnostic Test Wins FDA Clearance - Research Horizons

Ulcer-associated cell lineage expresses genes involved in regeneration and is hallmarked by high neutrophil gelatinase-associated lipocalin (NGAL) levels. - Abstract - Europe PMC