Mobile Workshop Supplies Pty Ltd Moonah TAS, 41% OFF
Why do the spectral lines in the hydrogen atom become closer together farther away from the nucleus? - Quora
Write Rydberg's formula for wavelength of the spectral lines of hydrogen atom spectrum. Mention to which series in the emission spectrum of hydrogen, H_alpha line belongs? - Quora
How many spectral lines are produced in the spectrum of a hydrogen atom from the 5th energy level? - Quora
Which hydrogen series has the longest wavelength? - Quora
How can a hydrogen atom, which has only one electron, have so many spectral lines? - Quora

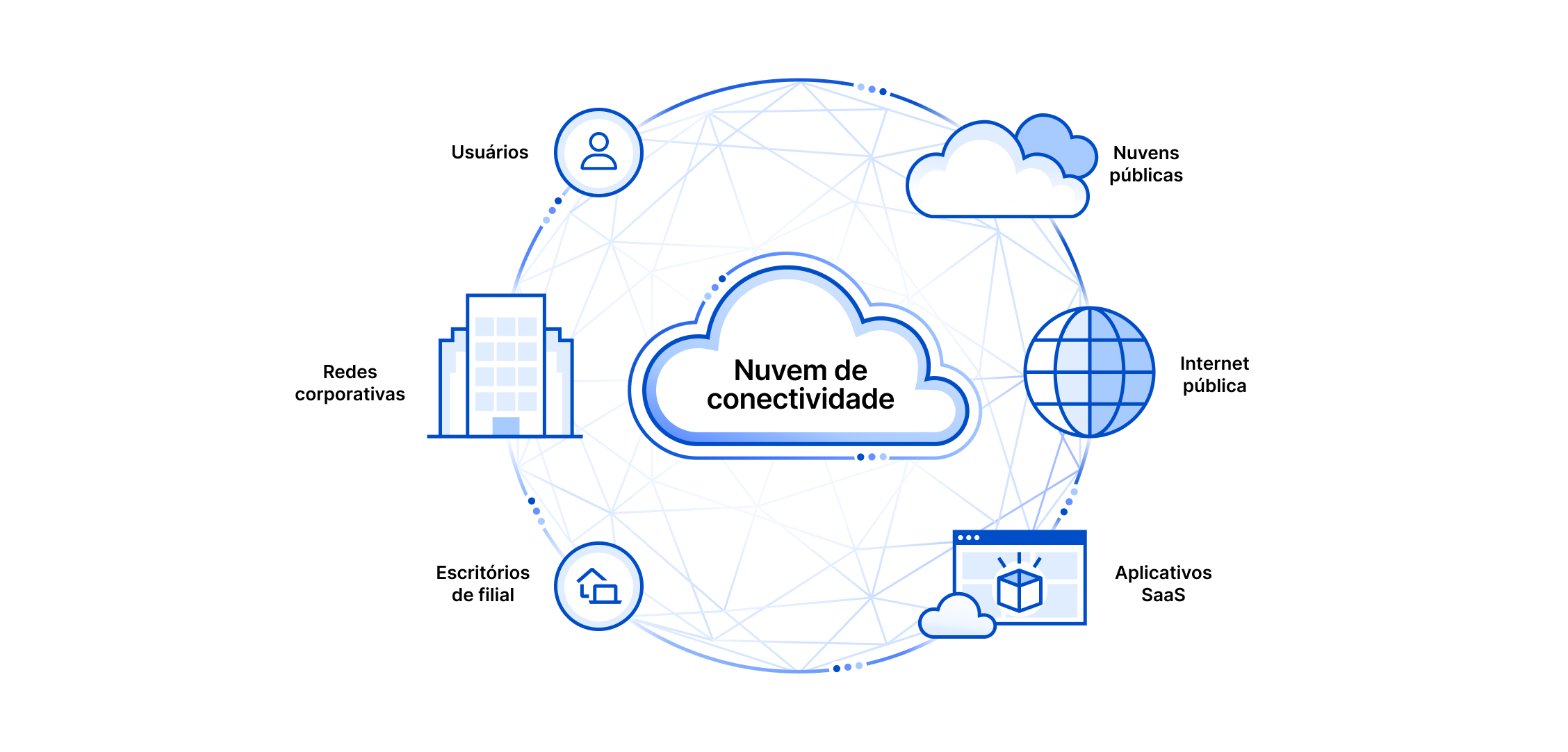
Quantum Mechanics in Three Dimensions

Mobile Workshop Supplies Pty Ltd Moonah TAS, 41% OFF
Why is hydrogen spectrum not a continuous spectrum? - Quora
How does the emitted spectra of an element depend on the structure of an atom of an element? - Quora
How can a hydrogen atom, which has only one electron, have so many spectral lines? - Quora
What are spectral lines? - Quora
What is the reason for spectral lines in the hydrogen spectrum? How do we explain it? - Quora
Why are the spectral lines of deuterium shifted to a shorter wavelength than those of ordinary hydrogen are? - Quora
What is the calculated energy (in kJ) of light emitted in the hydrogen spectrum, when an electron falls from the 4th energy level to the 3rd energy level? - Quora