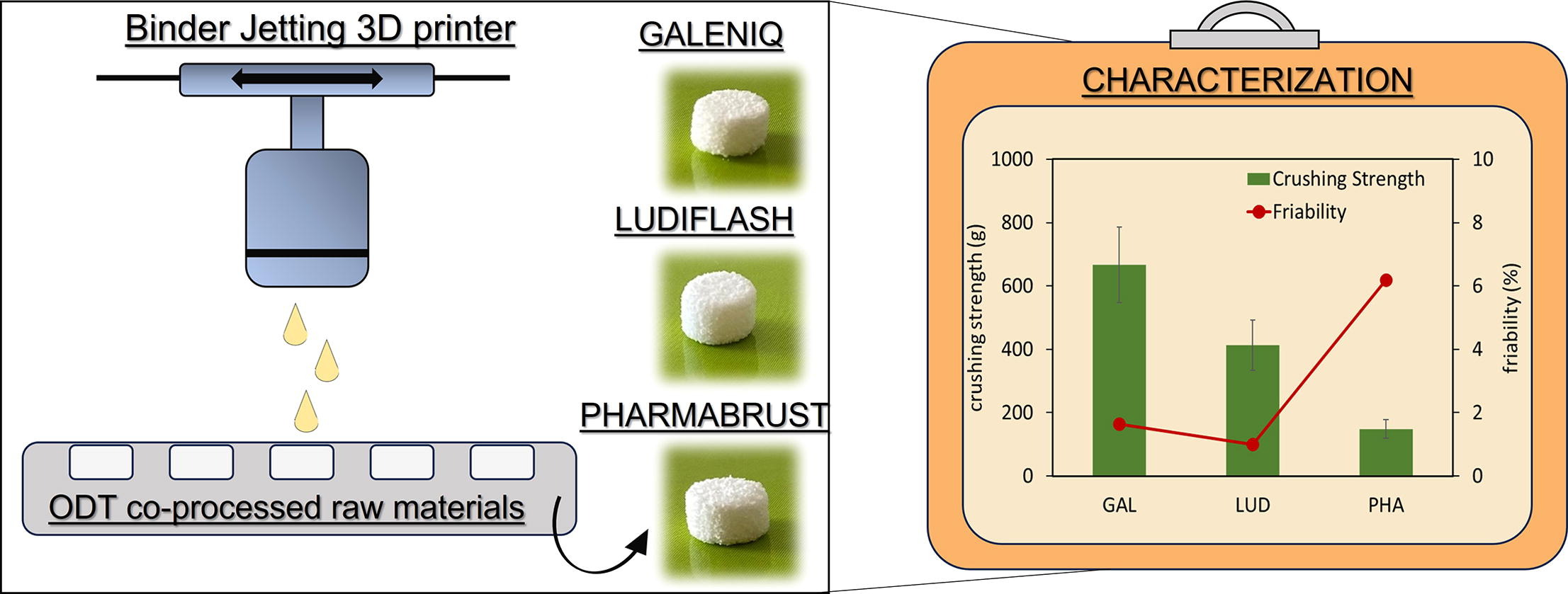
The study aimed to estimate the use of commercially available co-processed excipients, conventionally applied in compression protocols.
The use of co-processed materials for Orally Disintegrating Tablets (ODT) preparation by direct compression is well consolidated. However, the evaluation of their potential for ODT preparation by 3D printing technology remains almost unexplored. The

An update on microcrystalline cellulose in direct compression: Functionality, critical material attributes, and co-processed excipients - ScienceDirect

Orally disintegrating mini-tablets (ODMTs) – A novel solid oral dosage form for paediatric use - ScienceDirect

Saliha MOUTAHARRIK, PostDoc Position, Bachelor of Industrial Pharmacy, University of Milan, Milan, UNIMI, Department of Pharmaceutical Sciences (DISFARM)

Benefits of Co-processed High-Functionality Excipients in a Continuous Direct Compression Process - 2nd APV Continuous Manufacturing Conference - Pharma Excipients

PDF) Innovative Color Jet 3D Printing of Levetiracetam Personalized Paediatric Preparations

Co-processed materials testing as excipients to produce Orally Disintegrating Tablets (ODT) using binder jet 3D-printing technology - Pharma Excipients

Co processed excipient
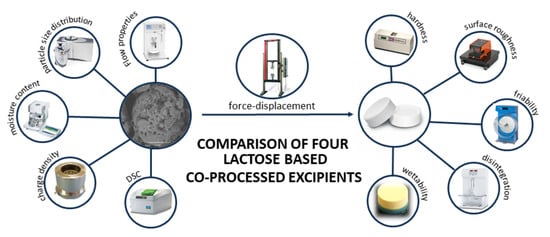
Pharmaceutics, Free Full-Text

SPECIAL FEATURE - Excipients: Manufacturers Look to Co-Processing as a Way of Improving Functionality

Saliha MOUTAHARRIK, PostDoc Position, Bachelor of Industrial Pharmacy, University of Milan, Milan, UNIMI, Department of Pharmaceutical Sciences (DISFARM)

Miriam COLOMBO, Professor (Assistant), Doctor of Philosophy, Università degli Studi di Milano-Bicocca, Milan, UNIMIB, Department of Biotechnology and Biosciences