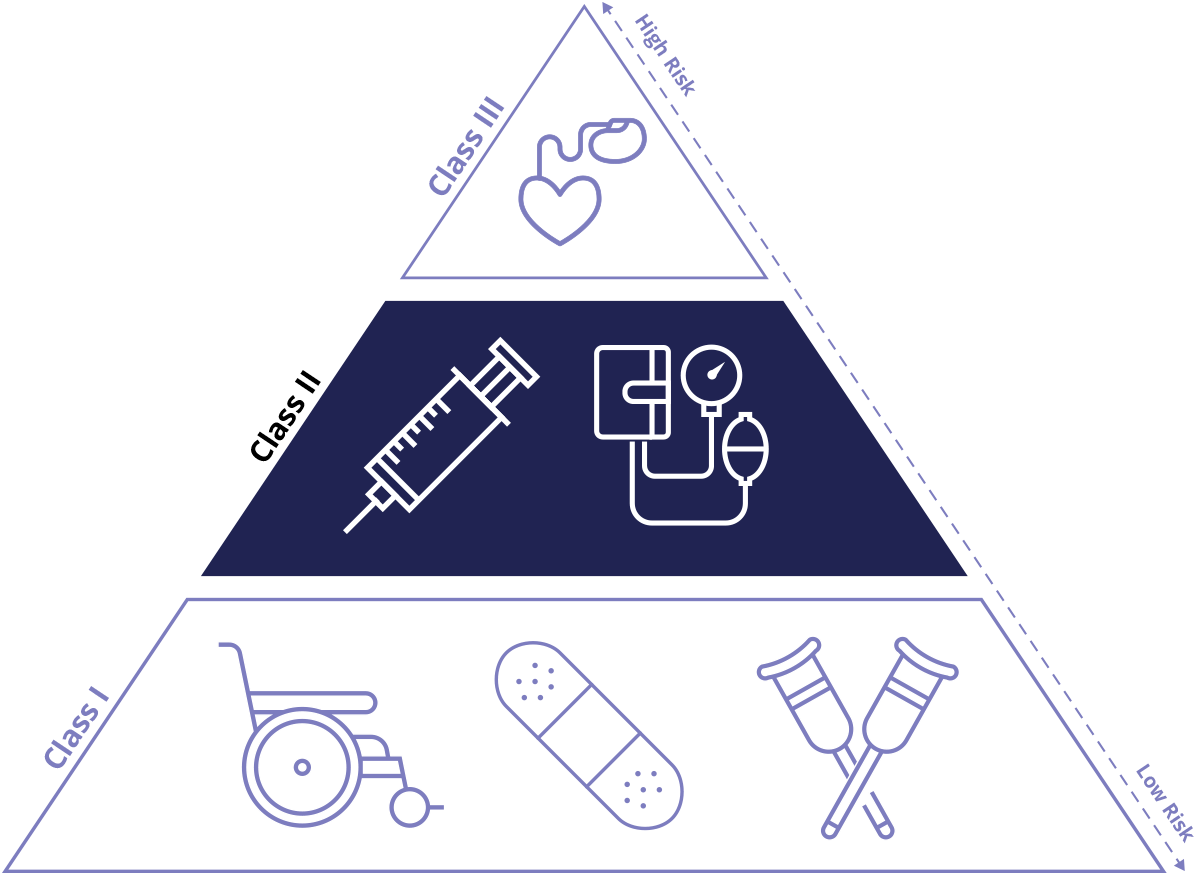
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.
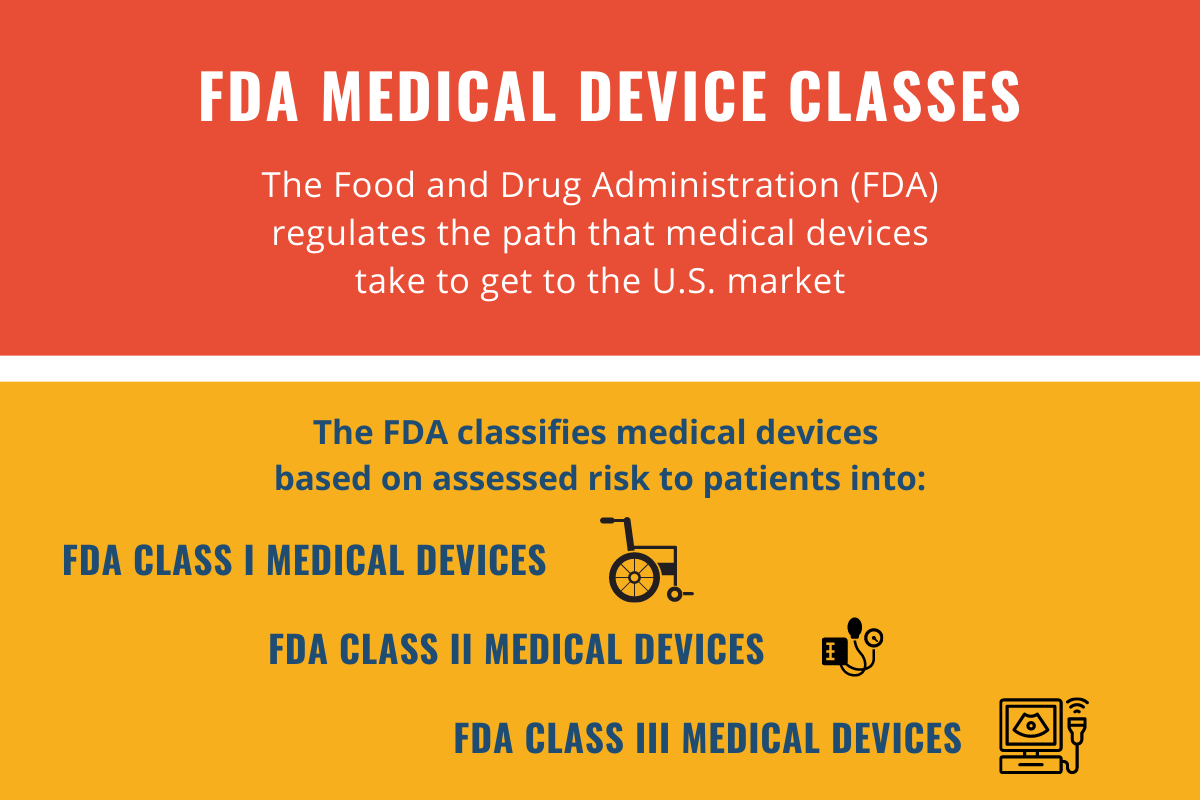
What Are the Three FDA Classes for Medical Devices? - RAM Technologies

SOCRA CCRP Exam - Term: Definition: What is in 21 CFR Part 11

Never accept the mark of the beast

Medical device regulations, classification & submissions

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials
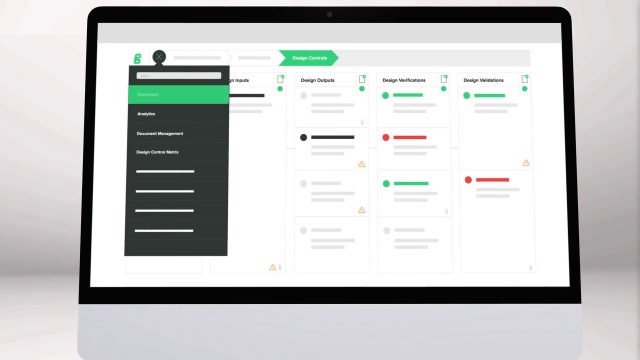
What is a Class 2 Medical Device in the US?

What's the Difference between a Class I Medical Device and a Class II?
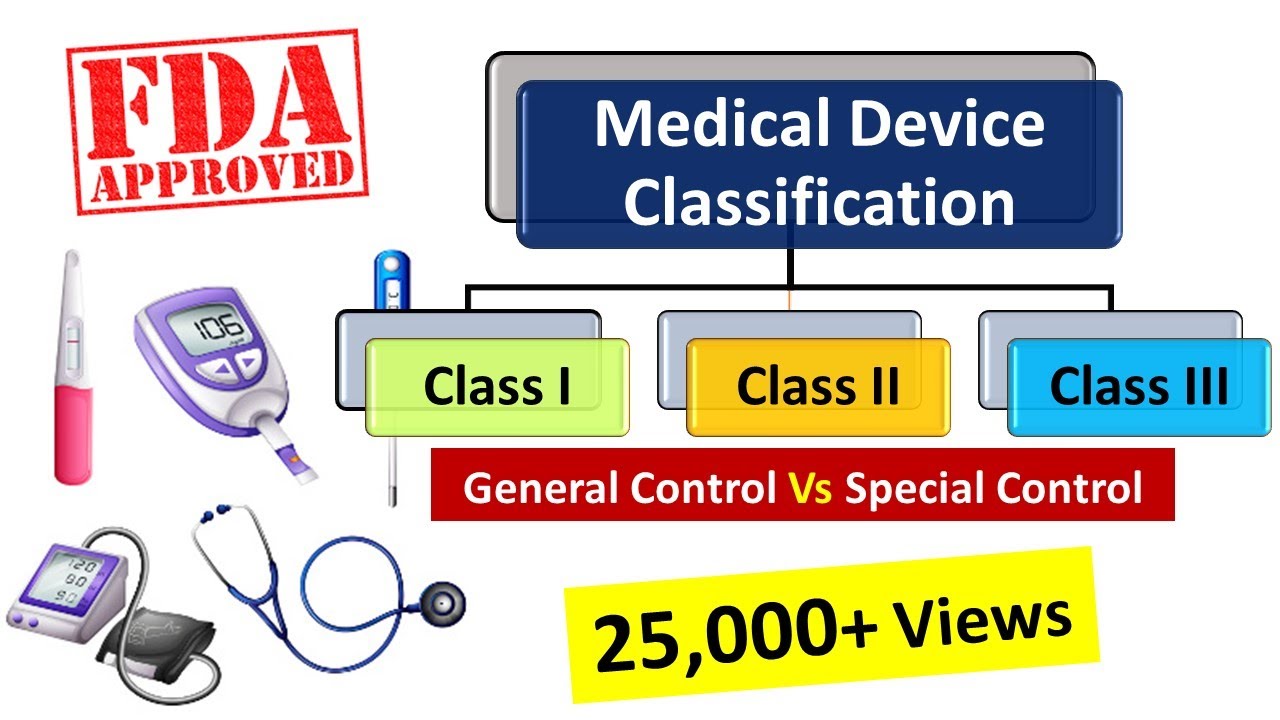
Medical Devices classification as per FDA, Medical Device Regulations
The 3 FDA medical device classes: differences and examples explained