2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1
CH104: Chapter 7 - Solutions - Chemistry

2.22At300 K,36 g of glucose present in a litre of its solution has an osm..

What is the molarity of a solution in which 18 grams of glucose with a molecular weight of 180 is dissolved in 500 grams of water? - Quora

ANSWERED] The boiling point of a solution is higher than the boiling - Kunduz

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

please explain the question and tell me what is 4 98 bar in this question and why it's not been - Chemistry - Solutions - 14451181
What is the molarity of a solution containing 16 grams of glucose for a 300 ml solution? - Quora
An aqueous solution of glucose containing 12 g in 100 g of water was found to boil at 100.34°C. Calculate Kh for water - Sarthaks eConnect

SOLVED: At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars

At S.T.P., the density of nitrogen monoxide is - (1) 3.0 GL-1 (2) 30 GL-1 (3) 1.34 L-1 (4)2.68 gL-1

At 300K 36 g of glucose present per litre in its solution has an osmotic pressure of 4 98 bar - Chemistry - Solutions - 12917865
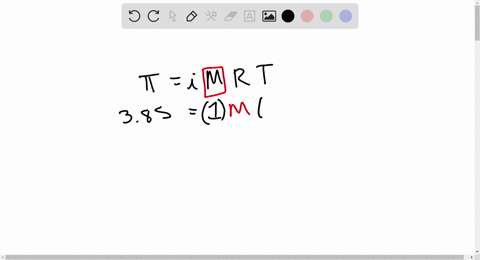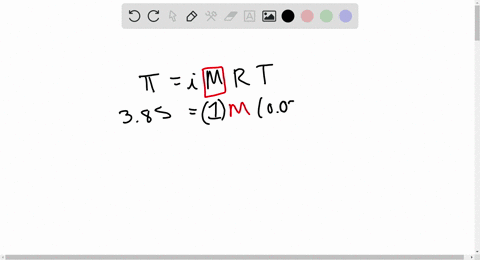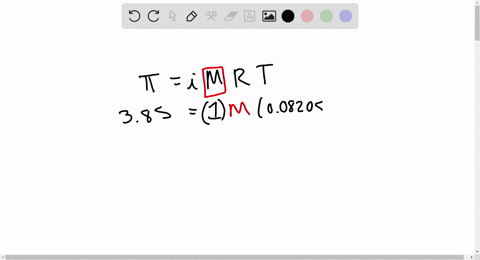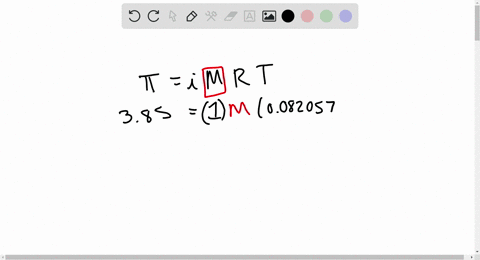
⏩SOLVED:At 300 K, 36 g of glucose present per litre in its…

is U. 2 If solubility of gas X (1) 0.5 gl. lity of gas 'X' is 0.5 gl- 1 bar then its solubility 3 bar pressure will be (2) 1.5 GL- (3)

Board Que 2023, PDF, Coordination Complex

2) 35.0 II. (1) 22.4 lit. (3) 448 lit. (4) 44800 ml Toleo At S.T.P., the density of nitrogen monoxide is - (2) 30 GL-1 (1)3.0 GL-1 (3) 1.34 gl-1 (4) 2.68 gL-1
:max_bytes(150000):strip_icc():focal(749x0:751x2)/bre-tiesi-74072fdf67c84e698a1039a77c6adc79.jpg)






