

Solved 6. The density of water at 0∘C is 0.9999 g/mL, and
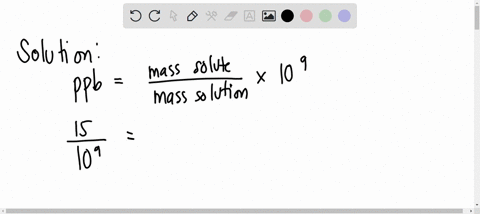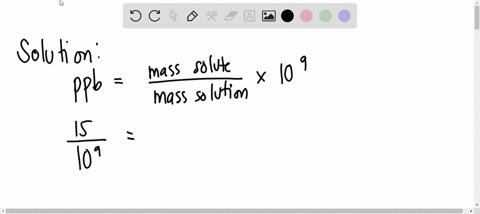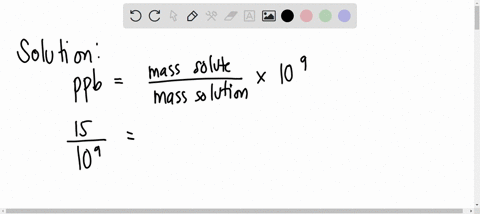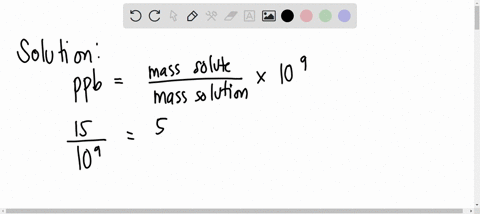
SOLVED: A water sample contains the pollutant chlorobenzene with a concentration of 17 ppb (parts per billion) by mass. What volume of this water contains 5.41×10^2 mg of chlorobenzene? (Assume a density
What is the volume of a solution, in mL, of sucrose, (C12H22O11
If oil is heavier than water, how does it float on top of water? Why doesn't gravity affect the heavier substance? - Quora

Enthalpy Calculations and Hess's Law, Practice Exam 2.2

Solved Assuming that the density of water is 1.00 g'ml., how

Biology density

Solved The density of water is 1.00 g/mL at 4°C. How many

17-11) The density of water at 4°C is 1.00 X 103 kg/m3. What is water's density at 94°C? Assume a c

Conversion from Molarity to ppm for a dilute solution

Addition of polyvinyl pyrrolidone during density separation with sodium iodide solution improves recovery rate of small microplastics (20–150 μm) from soils and sediments - ScienceDirect

17-11) The density of water at 4°C is 1.00 X 103 kg/m3. What is water's density at 94°C? Assume a c
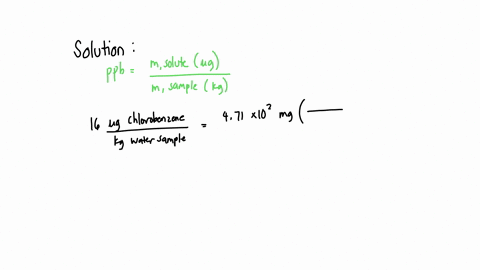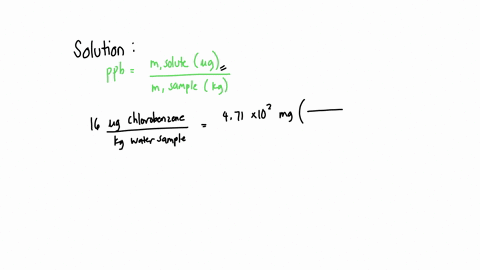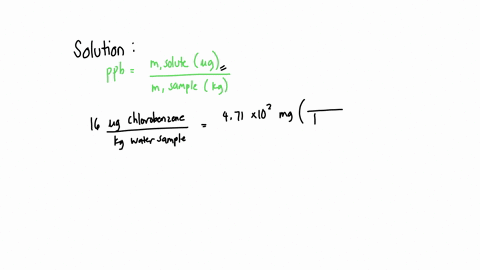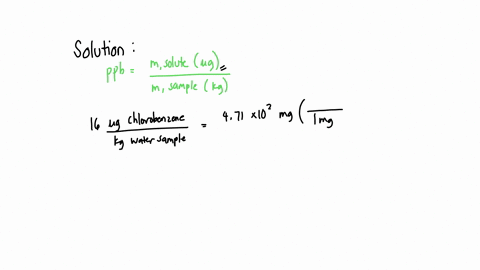
SOLVED: A water sample contains the pollutant chlorobenzene with a






